Abstract
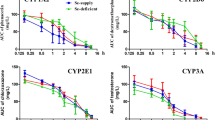
The expression of phase I and II biotransformation enzymes was examined with respect to experimental diet composition and with the addition of the bi-functional inducer flavone. Enzymatic activity and mRNA levels of cytochrome P450 monooxygenase (CYP) isoforms (CYP1A1, CYP1A2, CYP2B1/2) and glutathione-S-transferase (GST) isoforms (GSTA, GSTM, and GSTP) were used as indices for the changes in expression. An amino acid based (AA) diet and a semi-purified egg white (EW) diet were designed to include similar levels of nutrients and were compared to a standard laboratory chow (SC) diet. Rats (Sprague-Dawley) and mice (C57BL/6) were used as animal models. Animals were fed one of the three diets for 7 days prior to incorporation of flavone (2%, wt/wt). Diets with or without flavone were next fed for an additional 3 days. Enzymatic activities of the CYPs in mice and GSTs in both mice and rats were determined. In mice, the relative mRNA levels for each of the CYP and GST isoforms were also measured. The increase in phase I and II enzyme expression observed in response to flavone was most dynamic when the AA-based diet was used (often >20-fold for given isoform enzymatic activities and >200-fold for specific mRNAs), followed by the EW diet (10 to 20-fold and 100 to 200-fold, respectively). The SC diet resulted in a higher level of background expression of CYP and GST isoforms and as a consequence the observed fold increases in CYP and GST isoforms (enzymatic and mRNA levels) were substantially less (1 to 10-fold and 1 to 150-fold. respectively), when the SC diet fed group with or without flavone was compared.



Similar content being viewed by others
References
Bauerly KA, Storms DH, Harris CB, Hajizadeh S, Sun MY, Cheung CP, Satre MA, Fascetti AJ, Tchaparian E, Rucker RB (2006) Pyrroloquinoline quinone nutritional status alters lysine metabolism and modulates mitochondrial DNA content in the mouse and rat. Biochim Biophys Acta 1760:1741–1748
Benson AM, Hunkeler MJ, York JL (1989) Mouse hepatic glutathione transferase isoenzymes and their differential induction by anticarcinogens. Specificities of butylated hydroxyanisole and bisethylxanthogen as inducers of glutathione transferases in male and female CD-1 mice. Biochem J 261:1023–1029
Bounous G, Pageau R, Regoli D (1978) Enhanced 5-FU mortality in rats eating defined formula diets. Int J Clin Pharmacol Biopharm 6:265–267
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Burns SA, Hong YJ, Mitchell AE (2004) Direct liquid chromatography–mass spectrometry method for the detection of glutathione S-transferase isozymes and investigation of their expression in response to dietary flavone. J Chromatogr B Analyt Technol Biomed Life Sci 809:331–337
Butler LE, Dauterman WC (1989) Sensitivity of selected drug biotransformation enzymes to dietary protein levels in adult F334 male rats. J Biochem Toxicol 4:71–72
Canivenc-Lavier M-C, Vernevaut M-F, Totis M, Siess M-H, Magdalou J, Suschetet M (1996a) Comparative effects of flavonoids and model inducers on drug-metabolizing enzymes in rat liver. Toxicol 114:19–27
Canivenc-Lavier M-C, Bentejac M, Miller M-L, Leclerc J, Siess M-H, Latruffe M, Suschetet M (1996b) Differential effects of nonhydroxylated flavonoids as inducers of cytochrome P450 1A and 2B isozymes in rat liver. Toxicol Appl Pharmacol 114:348–353
Chanas SA, Jiang Q, McMahon M, McWalter GK, McLellan LI, ESCombe CR, Henderson CJ, Wolf CR, Moffat GJ, Itoh K, Yamamoto M, Hayes JD (2002) Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the glutathione S-transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 genes in the livers of male and female mice. Biochem J 365:405–416
Chen C, Kong A-N (2004) Dietary chemopreventive compounds and ARE/EpRE signaling. Free Radic Biol Med 36:1505–1516
Ciolino HP, Daschner PJ, Yeh GC (1999) Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem J 340:715–712
Di Simplico P, Jensson H, Mannervik B (1989) Effects of inducers of drug metabolism on basic hepatic forms of mouse glutathione transferase. Biochem J 263:679–685
Evers WD, Hook JB, Bond JT (1982) Alteration of toxicity to paraquat in mice fed a purified or cereal-based diet. Drug Nutr Interact 1:237–248
Gans JH (1982) Dietary influences on theobromine-induced toxicity in rats. Toxicol Appl Pharmacol 63:312–320
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hayes JD, Pulford DJ (1995) The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol 30:445–600
Hietanen E, Ahotupa M, Heikela A, Laitinen M (1982) Dietary cholesterol-induced changes of xenobiotics metabolism in liver. II Effects of phenobarbitone and carbon tetrachloride on activities of drug-metabolizing enzymes. Drug Nutr Interact 1:313–327
Ioannides C (1999) Effect of diet and nutrition on the expression of cytochromes P450. Xenobiotica 29:109–154
Kim M-J, Koh E, Surh J, Lee Kim Y-K, Kwon H (2002) Distribution of isoflavones and coumestrol in legumes and their products consumed in Korea. Food Sci Biotechnol 12:278–284
Kong AN, Owuor E, Yu R, Hebbar V, Chen C, Hu R, Mandlekar S (2001) Induction of xenobiotic enzymes by the MAP kinase pathway and the antioxidant or electrophile response element (ARE/EpRE). Drug Metab Rev 33:255–271
Martignoni M, de Kanter R, Grossi P, Saturno G, Barbaria E, Monshouwer M (2006) An in vivo and in vitro comparison of CYP gene induction in mice using liver slices and quantitative RT-PCR. Toxicol In Vitro 20:125–131
Merken HM, Beecher GR (2000) Liquid chromatographic method for the separation and quantification of prominent flavonoid aglycones. J Chromatogr A 897:177–184
Mitchell AE, Morin D, Lame MW, Jones AD (1995) Purification, mass spectrometric characterization, and covalent modification of murine glutathione S-transferases. Chem Res Toxicol 8:1054–1062
Mitchell AE, Burns SA, Rudolf JL (2007) Isozyme- and gender-specific induction of glutathione S-transferases by flavonoids. Arch Toxicol 81:777–784
Moon YJ, Wang X, Morris ME (2006) Dietary flavonids: effects on xenobiotics and carcinogen metabolism. Toxicol In Vitro 20:187–210
National Research Council (1995) Nutritional requirements for laboratory animals, 4th edn. National Academy Press, Washington DC, pp 1–174
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Paige RC, Royce FH, Plopper CG, Buckpitt AR (2000) Long-term exposure to ozone increases acute pulmonary centriacinar injury by 1-nitronaphthalene: I. Region-specific enzyme activity. J Pharmacol Exp Ther 295:934–941
Reeves PH (1993) AIN-93 purified diets for laboratory rodents—final report of the American Institution of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Reszka E, Wasowicz W, Gromadzinska J (2006) Genetic polymorphism of xenobiotic metabolising enzymes, diet and cancer susceptibility. Br J Nutr 96:609–619
Rettie AE, Williams FM, Rawlins MD, Mayer RT, Burke MD (1986) Major differences between lung, skin and liver in the microsomal metabolism of homologous series of resorufin and coumarin ethers. Biochem Pharmacol 35:3495–3500
Ritskes-Hoitinga M, Strubbe JH (2004) Nutrition and animal welfare. In: Kaliste E (eds) The welfare of laboratory animals. Kluwer Academic Publishers, Dordrecht, pp 1–363
Rosenberg DW (1991) Dietary modulation of cytochrome P450 in the small intestinal epithelium. Pharmacology 43:36–46
Steinberg F, Stites T, Anderson P, Storms D, Chan I, Eghbali S Rucker RB (2003) Pyrroloquinoline quinone improves growth and reproductive performance in mice fed chemically defined diets. Exp Biol Med 228:116–160
Stites T, Storms D, Bauerly K, Mah J, Harris C, Fascetti A, Rogers Q, Tchaparian E, Satre M, Rucker RB (2006) Pyrroloquinoline quinone modulates mitochondrial quantity and function in mice. J Nutr 136:390–396
Sun B, Fukuhara M (1997) Effects of co-administration of butylated hydroxytoluene, butylated hydroxyanisole and flavonoids on the activation of mutagens and drug-metabolizing enzymes in mice. Toxicology 26:61–72
Wattenberg LW (1975) Effects of dietary constituents on the metabolism of chemical carcinogens. Cancer Res 35:3326–3331
Wiwi CA, Gupte M, Waxman DJ (2004) Sexually dimorphic P450 gene expression in liver-specific hepatocyte nuclear factor 4alpha-deficient mice. Mol Endocrimol 18:1975–1987
Acknowledgments
We thank Dexter Morin for his assistance with the CYP analysis and Calliandra Harris for her assistance with the animal care and handling. Financial support was provided in part by a grant from the California Dairy Research Foundation, the Center for Health and Nutrition Research at UC Davis (http://chnr.ucdavis.edu/), and a gift from Mitsubishi Gas Chemical Company (Biochemical Division), Inc., Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rudolf, J.L., Bauerly, K.A., Tchaparian, E. et al. The influence of diet composition on phase I and II biotransformation enzyme induction. Arch Toxicol 82, 893–901 (2008). https://doi.org/10.1007/s00204-008-0310-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-008-0310-1