Abstract
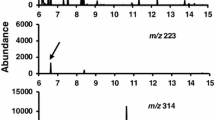
The elimination of tetryl was studied using ring-labeled 14C-tetryl. Tetryl was given subcutaneously to male Sprague–Dawley rats at doses of 25, 100, and 300 mg kg−1, and urine and feces were collected 24 h post-injection. Percent urinary elimination was observed to be 10.02 ± 2.48, 11.2 ± 1.66, and 13.24 ± 5.79 (mean ± SEM) respectively. Percent fecal elimination was 15.68 ± 6.13, 9.41 ± 1.52, and 8.45 ± 1.81 respectively. At 24 h post-injection, tissues from male Sprague–Dawley rats were collected from animals that received 100 mg kg−1 14C-tetryl. Tetryl was found to be poorly absorbed with approximately 65% of the administered dose remaining at the site of subcutaneous injection. Blood was found to be the principal depot of radioactivity, followed by muscle, liver, and kidney. Analysis of the tissue to blood radioactivity ratio revealed that the liver had the highest ratio (1.2), followed by brain (0.45), kidney (0.38), and testes (0.35). All other tissues analyzed had ratios less than 0.30. Urine of animals receiving 14C-tetryl (100 mg kg−1) was analyzed using HPLC coupled with UV detection (200–600 nm; 1.2 nm resolution). During HPLC analysis, 1 min fractions were collected and radioactivity measured. Two major peaks of radioactivity were identified at approximately 5 and 14 min retention times, respectively. The 14 min peak had the same retention time and UV spectrum as picric acid and 5 min peak had the same retention time and UV profile as picramic acid. The data presented demonstrates that that there is little retention of tetryl in specific tissue depots and that tetryl is eliminated in roughly equal amounts in both urine and feces. The major urinary metabolites identified picric acid and picramic acid (a known urinary metabolite observed in rabbits). From microsomal fraction studies, a major metabolite, NMPA, was identified. The formation of this metabolite was found to be dependent on at least two enzymes. One enzyme is dependent on NAD+ for NMPA formation and is likely to be NADP(H):quinone oxidoreductase. The second metabolite is NADP+ dependent and is probably related to NADPH:cytochrome-P450 reductase.



Similar content being viewed by others
References
Army (1987) Conventional weapons demilitarization: a health and environmental effects data base assessment: explosives and their co-contaminants. Frederick, MD. US Army Medical Research and Development Command, Fort Detrick. Document no. AD A220588
Bain WA, Thomson GH (1954) Pilot study of an antihistamine drug in control of “Tetryl” dermatitis. Br J Ind Med 11:25–30
Bergmann BB (1952) Tetryl toxicity: a summary of ten years experience. A.M.A. Arch Ind Hyg Occup Med 5:10
Brabham VW (1943) Tetryl illness in munition plants. J S C Med Assoc 39:193
Brownlie IA, Cumming WM (1946) Tetryl dermatitis: the interaction of aromatic nitro compounds with amino acids and proteins. Biochem J 40:640
Cave DA, Foster PMD (1990) Modulation of m-dinitrobenzene and m-nitrosonitrobenzene toxicity in rat sertoli-germ cell cocultures. Fundam Appl Toxicol 14:199–207
Daniele E (1964) Blood clotting alterations in chronic laboratory tetryl poisoning. Folia Medica 8:767–776
Eddy JH (1943) Some toxic reaction of common explosives. Indus Med 12:483–486
Gell PGH (1944) Sensitization to tetryl. Br J Exp Pathol 25:174
Gibbs T, Popolato A (1980) LASL explosive property data. University of California Press, Berkley
Griswold DP, Casey AE, Weisburger EK (1968) The carcinogenicity of multiple intragastric doses of aromatic and heterocyclic nitro or amino derivatives in young female Sprague–Dawley rats. Can Res 29:924–933
Guarino A, Zambrano A (1957) Histopathological research in subacute experimental intoxication by tetryl. Folia Medica 40:389–396
Hardy HL, Maloof CC (1950) Evidence of systemic effects of tetryl. Arch Ind Hyg 1:545–555
Harper CH, Llados F (1995) Toxicological profile for tetryl. Agency for toxic substances and disease registry, Atlanta, p 1
Hatch HS, Probst EW (1945) Effect of tetryl on the lungs. Ind Med 14:189–190
Hilton J, Swantson CN (1941) Clinical manifestations of tetryl and trinitrotoluene. Br Med J 2:509–510
Kirk-Othmer (1980). Encyclopedia of chemical technology, vol. 9. 3rd edn. Wiley, New York, p 585
Parmeggiani L, Bartalini E, Sassi C (1956) Tetryl occupational diseases: experimental investigation and prevention. Med Lavoro 47:293–313
Reddy T, Olson G, Weichman B, Reddy G, Torsella J, Daniel B, Leach G (1995) Toxicity of tetryl inF344 rats. Presentation at the annual meeting of the society of toxicology, Baltimore 1–25
Reddy G, Qualls C, Hampton A, Kim S (1997) Acute pathological and biochemical effects of tetryl in male rats. Res Commun Pharmacol Toxicol 2:1–11
Ruxton WL (1946) An investigation into the cause and prevention of industrial disease due to tetryl. Br J Dermatol 29:18–29
Troop HB (1946) Clinical effects of tetryl (CE powder). Br J Ind Med 3:20–21
Urbanski T (1967) Chemistry and technology of explosives, vol. 3. Pergamon. New York 40
Urbanski T (1984) Chemistry and technology of explosives, vol. 4. Pergamon. New York 370
Wells HG, Lewis JH, Sansum WD (1920) Observations of the toxicity of tetranitromethylanaline(tetryl), tetranitroxylene (TNX.), tetranitroanaline (TNA), dinitrochlorobenzene (Parazol), and metantraniline. J Ind Hyg 2:247–252
Witowski HJ, Fischer CN, Murdock HD (1942) Industrial illness due to tetryl. JAMA 119:1406–1409
Wyman J, Serve M, Hobson D (1992) Acute toxicity, distribution, and metabolism of 2,4,6-trinitrophenol (picric acid) in Fischer344 rats. J Toxicol Environ Health 37:313–327
Yinon J (1990) Toxicity and metabolism of explosives. CRC Press, Boca Raton, pp 69–80
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Myers, S.R., Spinnato, J.A. Tissue distribution and elimination of N-methyl-N-2,4,6-tetranitroaniline (tetryl) in rats. Arch Toxicol 81, 841–848 (2007). https://doi.org/10.1007/s00204-007-0220-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-007-0220-7