Abstract
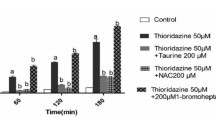
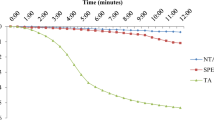
The cytotoxic effects of fenfluramine, an appetite suppressant, and its N-nitroso derivative, N-nitrosofenfluramine, have been studied in freshly isolated rat hepatocytes and isolated hepatic mitochondria. Exposure of hepatocytes to N-nitrosofenfluramine caused not only concentration (0.25–1.0 mmol L−1) and time (0–3 h)-dependent cell death accompanied by the loss of cellular ATP, adenine nucleotide pools, reduced glutathione (GSH), and protein thiols, but also the accumulation of oxidized glutathione and malondialdehyde (MDA), indicating lipid peroxidation. There was a time lag for the onset of the accumulation of MDA after the rapid depletion of ATP. Supplementation of the hepatocyte suspensions with N-acetylcysteine (4 mmol L−1), a precursor of intracellular GSH, partially inhibited N-nitrosofenfluramine (1 mmol L−1)-induced cytotoxicity. In comparative effects based on cell viability and rhodamine 123 retention, an index of mitochondrial membrane potential, fenfluramine was less toxic than N-nitrosofenfluramine. In mitochondria isolated from rat liver, N-nitrosofenfluramine caused an increase in the rate of state-4 oxygen consumption, indicating an uncoupling effect, and a decrease in the rate of state-3 oxygen consumption in a concentration-dependent manner. These results indicate that (a) mitochondria are target organelles for N-nitrosofenfluramine, which elicits cytotoxicity through mitochondrial dysfunction related to membrane potential and/or oxidative phosphorylation at an early stage and subsequently lipid peroxidation at a later stage; and (b) the toxicity of N-nitrosofenfluramine is greater than that of fenfluramine, suggesting participation of the nitroso group in the toxicity.



Similar content being viewed by others
Abbreviations
- DMSO:
-
Dimethyl sulfoxide
- GSH:
-
Glutathione
- GSSG:
-
Glutathione oxidized forms
- HEPES:
-
N-(2-hydroxyethyl)-piperazine-N-(2-ethanesulfonicacid)
- MDA:
-
Malondialdehyde
References
Adachi M, Saito H, Kobayashi H, Horie Y, Kato S, Yoshioka M, Ishii H (2003) Hepatic injury in 12 patients taking the herbal weight loss aids Chaso or Onshido. Ann Internal Med 139:488–492
Albano EM, Rundgren M, Hervison PJ, Nelson SD, Moldéus P (1985) Mechanism of N-acetyl-p-benzoquinone imine cytotoxicity. Mol Pharmacol 28:306–311
Archer MC, Labuc GE (1985) Nitrosamines. In: Anders MW (ed) Bioactivation of foreign compounds. Academic, New York, pp 403–431
Bellomo G, Mirabelli F, Richelmi P, Malorni W, Iosi F, Orrenius S (1990) The cytoskeleton as a target in quinone toxicity. Free Rad Res Comms 8:391–399
Brenot F, Herve P, Petiprez P, Parent F, Duroux P, Simonneau G (1993) Primary pulmonary hypertension and fenfluramine use. Br Heart J 70:537–541
Cain K, Skilleter DS (1987) Preparation and use of mitochondria in toxicological research. In: Snell K, Mullock B (eds) Biochemical toxicology, a practical approach. IRL Press, Oxford, pp 217–254
Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwords WD, Schaff HV (1997) Valvular heart disease associated with fenfluramine-phentermine. New Engl J Med 337:581–588
Corns S, Metcalfe K (2002) Risk associated with herbal slimming remedies. J Royal Soc Promot Health 122:213–219
Fawthrop DJ, Boobis AR, Davies DA (1991) Mechanism of cell death. Arch Toxicol 65:437–444
Fusi F, Sgaragli G, Murphy MP (1992) Interaction of butylated hydroxyanisole with mitochondrial oxidative phosphorylation. Biochem Pharmacol 43:1203–1208
Hansch C, Hoekman D, Leo A, Zhang L, Li P (1995) The expanding role of quantitative structure-activity relationship (QSAR) in toxicology. Toxicol Lett 79:45–53
Hinshaw DB, Sklar LA, Bohl B, Schraufstatter IU, Rossi MW, Spragg RG, Cochrane CG (1986) Cytoskeletal and morphological impact of cellular oxidant injury. Am J Pathol 123:454–464
Interactive LogKow. http://esc.syrres.com/interkow/kowdemo.htm
Irons RD, Sawahata T (1985) Phenols, Catechols, and Quinones. In: Anders MW (ed) Bioactivation of foreign compounds. Academic, New York, pp 259–281
Jones DP (1981) Determination of pyridine dinucleotides in cell extracts by high-performance liquid chromatography. J Chromatogr 225:446–449
Kanda T, Yokosuka O, Tada M, Kurihara T, Yoshida S, Suzuki Y, Nagano K, Saisho H (2003) N-Nitroso-fenfluramine hepatotoxicity resembling chronic hepatitis. J Gastroenter Hepato 18:999–1005
Kawata K, Takehira Y, Kobayashi Y, Kitagawa M, Yamada M, Hanajime K, Murohisa G, Kawamura M, Imaoka Y, Wada T, Morita S, Iwaizumi M, Makino S (2003) Three cases of liver injury by Sennomotokou, a Chinese dietary supplement for weight loss. Internal Med 42:1188–1192
Kehrer JP, Jones DP, Lemasters JJ, Farber H (1990) Contemporary issue in toxicology: mechanisms of hypoxic cell injury. Toxicol Appl Pharmacol 106:1907–1914
Lau G, Lo DST, Yao YJ, Lemong HT, Chan CE, Chu SS (2004) A fetal case of hepatic failure possibly induced by nitrosofenfluramine: a cases report. Med Sci Law 44:252–263
Lemasters JJ, Giuseppi JD, Nieminen AL, Herman B (1987) Blebbing, free Ca2+ and mitochondrial membrane potential preceding cell death in hepatocytes. Nature 325:78–80
Lemasters JJ, Nieminen AL, Chacon E, Imberti R, Gores G, Reece JM, Herman B (1993) Use of fluorescent probes to monitor mitochondrial membrane potential in isolated mitochondria, cell suspensions, and cultured cells. In: Lash LH, Jones DP (eds) Mitochondrial dysfunction. Academic, San Diego, pp 404–415
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Meylan WM, Howard PH (1995) Atom/fragment contribution method for estimating octanol-water partition coefficients. J Pharmaceut Sci 84:83–92
Moldéus P, Hogberg J, Orrenius S (1978) Isolation and use of liver cells. Methods Enzymol 52:60–71
Nakadai A, Inagaki H, Minami M, Takahashi H, Namme R, Ohsawa M, Ikegami S (2003) Determination of the optical purity of N-nitrosofenfluramine found in the Chinese slimming diet. Yakugaku Zasshi 123:805–809
Nakagawa Y, Moldéus P (1992) Cytotoxic effects of phenyl-hydroquinone and some hydroquinones on isolated rat hepatocytes. Biochem Pharmacol 45:1959–1965
Nakagawa Y, Moldéus P, Cotgreave IA (1992) The S-thiolation of hepatocellular protein thiols during diquat metabolism. Biochem Pharmacol 43:2519–2525
Nakagawa Y, Nakajima K, Tayama S, Moldéus P (1995) Metabolism and cytotoxicity of propyl gallate in isolated rat hepatocytes: effects of a thiol reductant and an esterase inhibitor. Mol Pharmacol 47:1021–1027
Nakagawa Y, Moldéus P (1998) Mechanism of p-hydroxybenzoate ester-induced mitochondrial dysfunction and cytotoxicity in rat hepatocytes. Biochem Pharmacol. 55:1907–1914
Nakagawa Y, Tayama S (2000) Metabolism and cytotoxicity of bisphenol A and other bisphenols in isolated rat hepatocytes. Arch Toxicol 74:99–105
Nicotera P, Bellomo G, Orrenius S (1992) Calcium-mediated mechanisms in chemically induced cell death. Annu Rev Pharmacol Toxicol 32:447–470
Nieminen AL, Gores GJ, Dawson TL, Herman B, Lemaster JJ (1990) Toxic injury from mercuric chloride in rat hepatocytes. J Biol Chem 265:2399–2408
Ottolenghi A (1959) Interaction of ascorbic acid and mitochondrial lipids. Arch Biochem Biophys 79:355–363
Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW (1980) High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide and related thiols and disulfides. Anal Biochem 106:55–62
Reed DJ (1986) Regulation of reductive processes by glutathione. Biochem Pharmacol 35:7–13
Reed DJ (1990) Glutathione: toxicological implications. Annu Rev Toxicol 30:603–631
Ricaurte GA, Molliver ME, Martello MB, Katz JL, Wilson MA, Martello AL (1991) Dexfenfluramine neurotoxicity in brains of non-human primates. Lancet 338:1487–1488
Rowland NE, Carlton J (1986) Neurobiology of an anorectic drug: fenfluramine. Prog Neurobiol 27:13–62
Sandy MS, Moldéus P, Ross D, Smith M (1986) Role of redox cycling and lipid peroxidation in bipyridyl herbicide cytotoxicity. Studies with a compromised isolated hepatocyte model system. Biochem Pharmacol 35:3095–3101
Schirmer RH, Krauth-Siegel RL, Schulz GE (1989) Glutathion reductase. In: Dolphin D, Poulson R. Avramovic O (eds) Glutathione: chemical, biochemical, and medical aspect. Part A, John Wiley, New York, pp 554–596
Thomas SH, Butt AY, Corris PA, Egan JJ, Higenbottam TW, Madden BP, Waller PC (1995) Appetite suppressants and primary pulmonary hypertension in the United Kingdom. Br Heart J 74:660–663
Thor H, Moldéus P, Orrenius S (1979) Metabolic activation and hepatotoxicity: effect of cysteine, N-acetylcysteine, and methionine on glutathione biosynthesis and bromobenzene toxicity in isolated rat hepatocytes. Arch Biochem Biophys 192:405–413
Thor H, Hartzell P, Orrenius S (1984) Potentiation of oxidative cell injury in hepatocytes which have accumulated Ca2+. J Biol Chem 259:6612–6615
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakagawa, Y., Suzuki, T., Kamimura, H. et al. N-Nitrosofenfluramine induces cytotoxicity via mitochondrial dysfunction and oxidative stress in isolated rat hepatocytes. Arch Toxicol 79, 312–320 (2005). https://doi.org/10.1007/s00204-004-0635-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-004-0635-3