Abstract
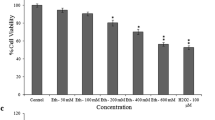
Recent studies point to an interaction between the glutamatergic neurotransmitter system and inorganic lead (Pb) neurotoxicity. Pb (1–100 µM) evoked cytotoxicity over the period of 72 h in mouse hypothalamic GT1-7 neurons. Glutamate (0.1 or 1 mM) on its own did not have any effect on cell viability. However, 1 mM glutamate clearly increased Pb-induced cell death at 48 and 72 h. Although flunarizine (0.1–10 µM), an antagonist of L- and T-type voltage-sensitive calcium channels (VSCCs), partially protected from the cytotoxicity induced by co-exposure to Pb (10 or 100 µM) and glutamate (1 mM), it had no protective effect on cytotoxicity induced by Pb alone. The flunarizine-induced protection was dependent on time and observed only at 48 h. Neither verapamil, an antagonist of L-type VSCCs, nor DIDS, an inhibitor of anion exchange, at non-toxic concentrations (0.1–10 µM) had any effect on cytotoxicity induced by Pb alone or together with glutamate at any studied time point. Co-exposure to Pb and glutamate also resulted in more prominent production of reactive oxygen species (ROS) than either of the compounds alone. Interestingly, we observed an increase in intracellular glutathione (GSH) levels in cells exposed to micromolar concentrations of Pb. Glutamate decreased the levels of intracellular GSH and also partially reduced the Pb-induced increase in GSH levels. These results suggest that the interaction of glutamate and Pb results in increased neuronal cell death via mechanisms that involve an increase in ROS production, a decrease in intracellular GSH defense against oxidative stress and probably T-type VSCCs.



Similar content being viewed by others
References
Adonaylo VN, Oteiza PI (1999) Lead intoxication: antioxidant defenses and oxidative damage in rat brain. Toxicology 135:77–85
Al-Modhefer AJA, Bradbury MWB, Simons TJB (1991) Observations on the chemical nature of lead in human blood serum. Clin Sci 81:823–829
Atlante A, Calissano P, Bobba A, Giannattasio S, Marra E, Passarella S (2001) Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett 497:1–5
Audesirk G (1993) Electrophysiology of lead intoxication: effects on voltage-sensitive ion channels. Neurotoxicology 14:137–147
Bannon DI, Olivi L, Bressler J (2000) The role of anion exchange in the uptake of Pb by human erythrocytes and Madin-Darby canine kidney cells. Toxicology 147:101–107
Battaini F (2001) Protein kinase C isoforms as therapeutic targets in nervous system disease states. Pharmacol Res 44:353–361
Berger R, Lehmann T, Karcher J, Garnier Y, Jensen A (1998) Low dose flunarizine protects the fetal brain from ischemic injury in sheep. Pediatr Res 44:277–282
Bressler J, Kim KA, Chakraborti T, Goldstein G (1999) Molecular mechanisms of lead neurotoxicity. Neurochem Res 24:595–600
Büsselberg D, Evans ML, Haas HL, Carpenter DO (1993) Blockade of mammalian and invertebrate calcium channels by lead. Neurotoxicology 14:249–258
Cai D, Mulle JG, Yue DT (1997) Inhibition of recombinant Ca2+ channels by benzothiazepines and phenylalkylamines: class-specific pharmacology and underlying molecular determinants. Mol Pharmacol 51:872–881
Ding Y, Gonick HC, Vaziri ND (2000) Lead promotes hydroxyl radical generation and lipid peroxidation in cultured aortic endothelial cells. Am J Hypertens 13:552–555
Domann R, Wunder L, Büsselberg D (1997) Lead reduces depolarization-induced calcium entry in cultured DRG neurons without crossing the cell membrane: fura-2 measurements. Cell Mol Neurobiol 17:305–314
Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649–671
Dubois EA, Zandbergen MA, Peute J, Goos HJ (2002) Evolutionary development of three gonadotropin-releasing hormone (GnRH) systems in vertebrates. Brain Res Bull 57:413–418
Finkelstein Y, Markowitz ME, Rosen JF (1998) Low-level lead-induced neurotoxicity in children: an update on central nervous system effects. Brain Res Rev 27:168–176
Gilbert ME, Lasley SM (2002) Long-term consequences of developmental exposure to lead or polychlorinated biphenyls: synaptic transmission and plasticity in the rodent CNS. Environ Toxicol Pharmacol 12:105–117
Goyer RA, Clarkson TW (2001) Toxic effects of metals. In: Klaassen CD (ed) Casarett and Doull's toxicology: the basic science of poisons, 6th edn. McGraw-Hill, New York, pp 811–867
Guilarte TR (1997) Glutamatergic system and developmental lead neurotoxicity. Neurotoxicology 18:665–672
Hiruma H, Uemura T, Kimura F (1997) Neuronal synchronization and ionic mechanisms for propagation of excitation in the functional network of immortalized GT1-7 neurons: optical imaging with a voltage-sensitive dye. J Neuroendocrinol 9:835–840
Hsu P, Liu M, Hsu C, Chen L, Guo Y (1997) Lead exposure causes generation of reactive oxygen species and functional impairment in rat sperm. Toxicology 122:133–143
Jadhav AL, Ramesh GT, Gunasekar PG (2000) Contribution of protein kinase C and glutamate in Pb2+-induced cytotoxicity. Toxicol Lett 115:89–98
Javors MA, King TS, Chang X, Ticku MK, Levinson C (1998) Characterization of chloride efflux from GT1-7 neurons: lack of effect of ethanol on GABAA response. Brain Res 780:183–189
Kobayashi T, Mori Y (1998) Ca2+ channel antagonists and neuroprotection from cerebral ischemia. Eur J Pharmacol 363:1–15
Lasley SM, Gilbert ME (2002) Rat hippocampal glutamate and GABA release exhibit biphasic effects as a function of chronic lead exposure level. Toxicol Sci 66:139–147
Legare ME, Barhoumi R, Burghardt RC, Tiffany-Castiglioni E (1993) Low-level lead exposure in cultured astroglia: identification of cellular targets with vital fluorescent probes. Neurotoxicology 14:267–272
Legare ME, Barhoumi R, Herbert E, Bratton GR, Burghardt RC, Tiffany-Castiglioni E (1998) Analysis of Pb2+ entry into cultured astroglia. Toxicol Sci 46:90–100
Lever SZ, Scheffel U (1998) Regional distribution of 203PbCl2 in the mouse after intravenous injection. Neurotoxicology 19:197–207
Loikkanen JJ, Naarala J, Savolainen KM (1998) Modification of glutamate-induced oxidative stress by lead: the role of extracellular calcium. Free Radic Biol Med 24:377–384
Mahesh VB, Zamorano P, De Sevilla L, Lewis D, Brann DW (1999) Characterization of ionotropic glutamate receptors in rat hypothalamus, pituitary and immortalized gonadotropin-releasing hormone (GnRH) neurons (GT1-7 cells). Neuroendocrinology 69:397–407
Malenka RC, Nicoll RA (1999) Long-term potentiation – a decade of progress? Science 285:1870–1874
Mazzolini M, Traverso S, Marchetti C (2001) Multiple pathways of Pb2+ permeation in rat cerebellar granule neurones. J Neurochem 79:407–416
Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI (1990) Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5:1–10
Naarala JT, Loikkanen JJ, Ruotsalainen MH, Savolainen KM (1995) Lead amplifies glutamate-induced oxidative stress. Free Radic Biol Med 19:689–693
Naarala J, Tervo P, Loikkanen J, Savolainen K (1997) Cholinergic-induced production of reactive oxygen species in human neuroblastoma cells. Life Sci 60:1905–1914
Nihei MK, Guilarte TR (2001) Molecular changes in glutamatergic synapses induced by Pb2+: association with deficits of LTP and spatial learning. Neurotoxicology 22:635–643
Nishizawa Y (2001) Glutamate release and neuronal damage in ischemia. Life Sci 69:369–381
Osborne NN, Wood JPM, Cupido A, Melena J, Chidlow G (2002) Topical flunarizine reduces IOP and protects the retina against ischemia-excitotoxicity. Invest Ophthalmol Vis Sci 43:1456–1464
Pauwels PJ, Leysen JE, Janssen PAJ (1991) Ca++ and Na+ channels involved in neuronal cell death. Protection by flunarizine. Life Sci 48:1881–1893
Sagara Y, Dargusch R, Chambers D, Davis J, Schubert D, Maher P (1998) Cellular mechanisms of resistance to chronic oxidative stress. Free Radic Biol Med 24:1375–1389
Savolainen KM, Loikkanen J, Eerikäinen S, Naarala J (1998a) Glutamate-stimulated ROS production in neuronal cultures: interactions with lead and the cholinergic system. Neurotoxicology 19:669–674
Savolainen KM, Loikkanen J, Eerikäinen S, Naarala J (1998b) Interactions of excitatory neurotransmitters and xenobiotics in excitotoxicity and oxidative stress: glutamate and lead. Toxicol Lett 102–103:363–367
Simons TJB (1993) Lead-calcium interactions in cellular lead toxicity. Neurotoxicology 14:77–85
Simons TJB, Pocock G (1987) Lead enters bovine adrenal medullary cells through calcium channels. J Neurochem 48:383–389
Somashekaraiah BV, Padmaja K, Prasad AR (1992) Lead-induced lipid peroxidation and antioxidant defense components of developing chick embryos. Free Radic Biol Med 13:107–114
Sortino MA, Aleppo G, Copani A, Casabona G, Nicoletti F, Ventra C, Kuhn R, Knöpfel T, Malitschek B, Canonico PL (1996) Immortalized hypothalamic neurons express metabotropic glutamate receptors positively coupled to cyclic AMP formation. Eur J Neurosci 8:2407–2415
Spergel DJ, Krsmanovic LZ, Stojilkovic SS, Catt KJ (1994) Glutamate modulates [Ca2+]i and gonadotropin-releasing hormone secretion in immortalized hypothalamic GT1-7 neurons. Neuroendocrinology 59:309–317
Striessnig J, Grabner M, Mitterdorfer J, Hering S, Sinnegger MJ, Glossmann H (1998) Structural basis of drug binding to L Ca2+ channels. Trends Pharmacol Sci 19:108–115
Tomsig JL, Suszkiw JB (1991) Permeation of Pb2+ through calcium channels: fura-2 measurements of voltage- and dihydropyridine-senstive Pb2+ entry in isolated bovine chromaffin cells. Biochim Biophys Acta 1069:197–200
Tytgat J, Pauwels PJ, Vereecke J, Carmeliet E (1991) Flunarizine inhibits a high-threshold inactivating calcium channel (N-type) in isolated hippocampal neurons. Brain Res 549:112–117
Urbanski HF, Fahy MM, Daschel M, Meshul C (1994) N-methyl-d-aspartate receptor gene expression in the hamster hypothalamus and in immortalized luteinizing hormone-releasing hormone neurones. J Reprod Fertil 100:5–9
Wiemann M, Schirrmacher K, Büsselberg D (1999) Interference of lead with the calcium release activated calcium flux of osteoblast-like cells. Calcif Tissue Int 65:479–485
Winder C (1993) Lead, reproduction and development. Neurotoxicology 14:303–317
Yang R-C, Shih H-C, Hsu H-K, Chang H-C, Hsu C (2003) Estradiol enhances the neurotoxicity of glutamate in GT1-7 cells through an estrogen receptor-dependent mechanism. Neurotoxicology 24:65–73
Zapater P, Moreno J, Horga JF (1997) Neuroprotection by the novel calcium antagonist PCA50938, nimodipine and flunarizine, in gerbil global brain ischemia. Brain Res 772:57–62
Acknowledgements
The authors wish to thank Ms. Virpi Koponen for her excellent technical assistance and Dr. Ewen MacDonald for revising the language of this manuscript. Ms. Maaret Heickell is thanked for her contribution to the experimental part of the measurements of ROS and the effect of different antagonists on cytotoxicity. This work was supported by the Emil Aaltonen Foundation, University of Kuopio, Finland and the Finnish Institute of Occupational Health. The research was conducted in accord with all relevant local legislation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loikkanen, J., Naarala, J., Vähäkangas, K.H. et al. Glutamate increases toxicity of inorganic lead in GT1-7 neurons: partial protection induced by flunarizine. Arch Toxicol 77, 663–671 (2003). https://doi.org/10.1007/s00204-003-0498-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-003-0498-z