Abstract.
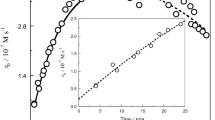
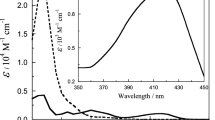
This in vitro study investigated the formation of hydroxyl radicals (•OH) under anaerobic conditions through the direct reaction between paraquat radicals (PQ+•) and hydrogen peroxide (H2O2) by quantitative UV-VIS and electron spin resonance (ESR) spectroscopy. PQ+• was formed by paraquat reduction using either sodium dithionite or the xanthine/xanthine oxidase reaction as electron donors. The anaerobic formation of PQ+• was quantified both by measuring light absorption at 605 nm or by ESR techniques respectively, using either the absorption coefficient or ultramarine as a stable spin standard. Detection of •OH took place with aid of the spin trap 5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide (DEPMPO). Generation or addition of H2O2 to PQ+• eliminates the 35-line ESR signal of PQ+• and subsequently generates the 8-line ESR signal of the DEPMPO-OH adduct. The elimination of PQ+• as well as the formation of OH-DEPMPO adduct was not influenced by 1.0 mM deferoxamine, indicating that iron or other transition metals are, at least under anoxic conditions, not necessarily involved in the generation of the most aggressive reactive oxygen species •OH.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Weidauer, .E., Mörke, .W., Foth, .H. et al. Does the anaerobic formation of hydroxyl radicals by paraquat monocation radicals and hydrogen peroxide require the presence of transition metals?. Arch Toxicol 76, 89–95 (2002). https://doi.org/10.1007/s00204-001-0303-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00204-001-0303-9