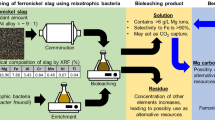
Abstract
In many European regions, both local metallic and non-metallic raw materials are poorly exploited due to their low quality and the lack of technologies to increase their economic value. In this context, the development of low cost and eco-friendly approaches, such as bioleaching of metal impurities, is crucial. The acidophilic strain Acidiphilium sp. SJH reduces Fe(III) to Fe(II) by coupling the oxidation of an organic substrate to the reduction of Fe(III) and can therefore be applied in the bioleaching of iron impurities from non-metallic raw materials. In this work, the physiology of Acidiphilium sp. SJH and the reduction of iron impurities from quartz sand and its derivatives have been studied during growth on media supplemented with various carbon sources and under different oxygenation conditions, highlighting that cell physiology and iron reduction are tightly coupled. Although the organism is known to be aerobic, maximum bioleaching performance was obtained by cultures cultivated until the exponential phase of growth under oxygen limitation. Among carbon sources, glucose has been shown to support faster biomass growth, while galactose allowed highest bioleaching. Moreover, Acidiphilium sp. SJH cells can synthesise and accumulate Poly-β-hydroxybutyrate (PHB) during the process, a polymer with relevant application in biotechnology. In summary, this work gives an insight into the physiology of Acidiphilium sp. SJH, able to use different carbon sources and to synthesise a technologically relevant polymer (PHB), while removing metals from sand without the need to introduce modifications in the process set up.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Extremophilic organisms are defined by their ability to thrive in harsh environmental conditions, regarding temperature, salinity, pressure, and pH, among others. These organisms populate hostile habitats and are endowed with ad hoc physiological and molecular features that allow adaptation to the specific environment (Rothschild and Mancinelli 2001).
Acidophiles can survive at the very low pH values found in natural environments or generated by human activities, such as mining. Indeed, many acidophilic bacteria have been isolated from mine drainage waters where pH values can approach 2 (Baker-Austin and Dopson 2007). In this context, some extreme acidophilic bacteria are described as capable of performing the dissimilatory reduction of ferric ion (from Fe3+ to Fe2+) which is relevant for bioleaching, that is the exploitation of the metabolic activities or metabolic products of specific microorganisms for the extraction of metals. Quartz sand (or silica sand), derived from the erosion of quartz rock, is largely used as a non-metallic raw material in industry, from low value applications such as glass manufacturing, foundry casting, ceramic, and adhesive filler to high-added value applications such as silicon-metal wafers, optical glasses and photovoltaic panels (Sajjad et al. 2019; Brierley 2010; Platias et al. 2014). A major problem in the use of quartz sand is the presence of sulphides and iron-oxide compounds, such as goethite, hematite, or siderite, which are considered impurities and indeed compromise the qualities and properties of the sand itself (Platias et al. 2014). This has fostered the development of technologies to reduce or eliminate the presence of ferrous compounds in the raw material (Zhang et al. 2012; Du et al. 2011; Panda et al. 2015a). With the aim to improve the technological properties of materials, physical (electromagnetic and gravitational separation) and chemical (leaching with inorganic and organic acids and reductants) methods of mineral processing have been applied. Since chemical-physical methods, e.g. hydrometallurgy are expensive and energy demanding, as well as seriously polluting water, air and soils (Veglio’ 1997; Brierley 2010; Asghari et al. 2013; Panda et al. a, b), bioleaching has become increasingly popular over the years as an economical, eco-friendly and sustainable method for leaching of impurities from raw materials (Bosecker 1997; D’Hugues et al. 2008; Anjum et al. 2010, 2012; Brierley 2010; Sajjad et al. 2019; Srichandan et al. 2019; Wang et al. 2020).
Candidate micro-organisms for bioleaching differ in metabolic requirements (chemolithotrophic or chemoorganotrophic), growth temperature (mesophiles or thermophiles), pH conditions (acidophiles or neutrophiles), and oxygen dependence (Clark and Norris 1996; Kelly and Wood 2000; Norris et al. 2000; Johnson and Bridge 2002; Ohmura et al. 2002; Rohwerder et al. 2003). In recent years, several genera of bacteria and archaea have been characterised as suitable for iron bioleaching purposes, including chemolithoautotrophic strains (i.e. Acidiothiobacillus sp.) and heterotrophic bacteria such as Acidiphilium (Harrison 1981; Malik and Hedrich 2022; Johnson and McGinness 1991a; Johnson and Bridge 2002). These acidophilic heterotrophic microorganisms are known to reduce Fe(III) to Fe(II) under different oxygenation conditions by coupling the oxidation of an organic substrate to the reduction of Fe(III) (Küsel et al. 1999; Li et al. 2020a; Bridge and Johnson 2000; Kupka et al. 2007; Vašková and Kupka 2007). Acidiphilium sp. SJH was isolated from an acidic mine in North Wales and shown to be able to reduce ferric ion under different conditions and to bioleach the iron fraction derived from quartz sand (Bridge and Johnson 2000; Kupka et al. 2007; Vašková and Kupka 2007; Johnson et al. 1979).
This work aims to address the metabolic abilities of this acidophilic bacterium, studying its growth kinetics and the reduction of ferric iron in quartz sand in the presence of different carbon sources and growth conditions. Moreover, Fourier-transform infrared spectroscopy analysis (FTIR) was performed on whole Acidiphilium sp. SJH cells to define if and how the process affects their cellular response and the overall macromolecular composition. This approach has been applied successfully to study changes induced by various stressors as for example the overproduction of recombinant proteins and protein aggregation (Doglia et al. 2008; DeDivitiis et al. 2023).
Materials & methods
Strains and materials
Acidiphilium sp. SJH (NCIMB 12,826) was kindly provided by Prof. David Barrie Johnson (Bangor University, UK) and maintained in autoclave-sterilised acidic liquid medium (pH 2.5) composed of tryptic soy broth 0.26 g/L, (NH4)2SO4 12.5 g/L, MgSO4·7H2O 5 g/L, galactose 2 g/L, salt mix 1 mL for 1 L medium. The composition of the salt mix was as follows: Na2HPO4 177 g/L, KH2PO4 170 g/L, NH4Cl 133 g/L, Na2SO4 35.5 g/L, ZnSO4·7 H2O 10 g/L, CuSO4·5 H2O 1 g/L, MnSO4·H2O 0.76 g/L, CoSO4·7 H2O 1 g/L.
The sample of aeolian quartz sand from Šajdíkove Humence deposit (west Slovakia), hereafter named “as received”, was kindly provided by KERKOSAND, Ltd together with information about grain size ranging from 0.3 to 1.4 mm (mean grain diameter d50 = 0.6 mm). Quartz sand was autoclave-sterilised before bioleaching experiments.
Furthermore, the heavy fraction of the raw material containing coloring oxides was separated by gravity using bromoform (CHBr3) and the light fraction was further treated by bacterial leaching. The light fraction still contained residual iron impurities in quantities that exceeded the limits for the input raw material for glass production. Chemical analyses of the quartz sand and the separated light fraction (Table 1) was done by Atomic Absorption Spectrometer (AAS Varian AA-30), after dissolving the solid sample in aqua regia using a microwave digestion system (Berghof).
Bacterial growth
Acidiphilium sp. SJH cultures, maintained in liquid broth, were inoculated at 0.1 OD600 in the above-mentioned medium supplemented with different carbon sources: galactose (2 g/L), glycerol (2 g/L) or glucose (2, 5–10 g/L) and incubated at 30 °C, shaking at 180 rpm, in fully aerobic conditions. Growth was monitored by measuring the optical density (OD600) for up to 14 days. Duplication time was estimated from OD600 measurements. First, µMAX was calculated as the slope of the regression line of ln (OD600) of samples in exponential phase; then tD was obtained as ln(2)/µMAX. All experiments were performed in triplicate.
Consumption of carbon sources was monitored over time for up to 14 days by High-Performance Liquid Chromatography (HPLC, Agilent 1100/1200, Agilent Technologies, Inc.). The instrument was equipped with a Rezex™ ROA-Organic Acid H+ (8%) 300 × 7.8 mm column (Phenomenex). The column was kept at 40 °C and 0.005 N H2SO4 was pumped isocratically at a flow rate of 0.5 mL/min. Signals were detected by a refractive index detector (RID) and peaks were identified by comparison with known standards. Before analysis, each sample was centrifuged at 13,000 g for 10 min, the pH was adjusted to neutrality with NaOH 0.2 M and filtered with a 0.22 μm syringe filter.
O2 consumption and CO2 production during bacterial growth were measured using on-line gas analysis. Cultures were grown in magnetically stirred and adequately aerated reaction vessels with a working volume of 0.5 L. Under fully aerobic conditions oxygen was continuously supplied by air sterilized by filtration through a 0.22 μm pore size filter passing through the reactor headspace above the liquid. On-line measurement of oxygen consumption and carbon dioxide production from the gas-phase was used to accurately measure oxidation of the organic substrate. For baseline concentration of zero CO2 and stable O2 (20.95% vol.), a source of compressed dry “zero air” was used. Air was directed to reactors through mass flow controllers (Bronkhorst) to maintain a constant standard temperature and pressure (STP)-corrected flow rate of the incoming air. To eliminate evaporative water loss from the cultures, the air entering the reactors was re-humidified using Nafion® tubing submerged in deionized water. The gas multiplexer (Sable Systems International) was used for automatic switching of the air flow between baseline air and headspace gas of individual reaction flasks. The exhausted gas was scrubbed again with water vapour by combined Nafion membrane/anhydrone drying before analysis. Paramagnetic oxygen analyser and infrared carbon dioxide analyser (Sable Systems) were used for O2 and CO2 analyses in inlet and outlet air respectively. The analysers were calibrated for zero (99.99% N2) and ambient air O2 and CO2 concentrations span. The bioreactor temperature was controlled by means of a circulating water bath thermostat.
Bioleaching of iron impurities from quartz sand
Leaching of quartz sand was carried out at 40% w/v final concentration in 100 mL flasks, testing different carbon sources, aeration conditions (microaerobic, aerobic, and full aerobic condition) and phase of growth of the bacterial culture (either exponential or stationary). Aeration of the cell cultures, adjusted according to the work of Johnson et al. (Johnson and McGinness 1991a; Johnson and Bridge 2002), was modulated through two parameters: the ratio between the culture volume and the total volume of the flask and agitation. For microaerobic cultures, cells were inoculated into 100 mL flasks filled with 100 mL of medium and quartz sand and incubated at 25 °C without shaking. In aerobic condition flasks contained 50 ml of the mix of quartz sand and cells culture and were incubated at 25 °C under 180 rpm shaking. The full aerobic condition was obtained with 20 mL culture incubated at 25 °C and 180 rpm shaking.
Exponential phase bioleaching was performed with cells pre-cultured under aerobic conditions, up to the exponential phase (0.5 OD600) and then inoculated into fresh medium with the same composition supplemented with 40% (w/v) quartz sand at 0.2 OD600. Stationary phase bioleaching was performed by pre-growing the cells under aerobic conditions up to the stationary phase (1.8 OD600). Subsequently, quartz sand was added up to 40% w/v.
Samples of the leaching liquors were taken in regular intervals through the cap’s septum by using sterile needles and syringes. The samples were filtered through membrane filters and immediately stabilized with sulphuric acid to avoid Fe2+ oxidation. Ferric iron concentration in the leaching liquors was determined by a UV-spectrophotometric method at 300 nm (Basaran and Tuovinen 1986). For ferrous iron detection, several colorimetric methods have been described (Yue et al. 2016; Bas 2021). Here, ferrous iron concentrations were determined by the modified o-phenanthroline spectrophotometric method, insensitive to Fe(III) interference (Herrera et al. 2007).
Whole- cell fourier-transform infrared spectroscopy (FTIR) analyses
Acidiphilium sp. SJH cells were cultured for two weeks under bioleaching conditions (microaerobic conditions, galactose medium, 40% quartz sand). Bacterial cell samples were collected at time 0 and after 1 and 2 weeks of growth, pelleted by centrifugation at 3,500 g for 5 min and washed in physiological solution (NaCl 0.9%). This washing step was repeated 3 times to remove any trace of medium components. Cells were then resuspended in an appropriate volume of physiological solution prior to FTIR measurements. Cell suspensions (3 µL) were deposited onto a BaF2 window, transparent to IR, and dried at room temperature for at least 30 min to eliminate the excess of water (Ami et al. 2022). FTIR absorption spectra were acquired in transmission mode, in the 4000–700 cm− 1 spectral range, by a Varian 610-IR infrared microscope coupled to the Varian 670-IR FTIR spectrometer (both from Varian Australia Pty Ltd., Mulgrave VIC, Australia) and equipped with a nitrogen-cooled mercury cadmium telluride detector (Ami et al. 2021, 2022). The variable microscope aperture was adjusted to ~ 200 μm × 200 μm (spatial resolution). Measurements were performed at 2.0 cm− 1 spectral resolution, 25 kHz scan speed, triangular apodization, and by the accumulation of 512 scan co-additions. Spectra were normalized to the Amide I band area and the second derivative analysis was performed (after a 13-point smoothing of the measured spectra) by the Savitzky–Golay method (3rd polynomial, 9 smoothing points), using the GRAMS/32 software (Galactic Ind. Corp., Salem, NH, USA). For each sample, several spectra were collected by selecting different areas on the same sample through the variable diaphragm aperture of the infrared microscope. The control condition was set up by cultivating Acidiphilium sp. SJH cells under bioleaching conditions but without quartz sand. To evaluate the reproducibility of the results, two independent experiments were performed.
Results
Microbial growth kinetics on different carbon sources
In medium containing glucose at 2 g/L, the culture showed a duplication time of 14.6 h and reached a maximum OD600 of 2.24 after 3 days (Fig. 1A). In galactose containing broth, the observed duplication time was 34.3 h and OD600 2.09 was reached in 6 days. In the medium added with glycerol the duplication time was 48.3 h and the highest density of 2.37 OD600 was measured after 9 days cultivation. The faint growth observed in minimal medium without any added carbon source was probably supported by carry-over of residual nutrients from the pre-culture.
As glucose turned out to be the more effective carbon source for growth, it was tested at higher concentrations to boost biomass accumulation. As shown in Fig. 1B, in 5 g/L glucose the culture grew with a duplication time of 14.3 h and reached a maximum OD600 of 2.6. In medium containing 10 g/L glucose, cells displayed a duplication time of 13.7 h and reached 2.9 OD600. At all glucose concentrations tested, the culture entered the stationary phase after the third day of growth.
HPLC analysis showed that 2 g/L glucose, galactose and glycerol were completely depleted at the growth peak (Fig. 1A and C). Accordingly, gas analysis of the culture grown in glucose medium showed a close coupling of oxygen consumption and carbon dioxide production, as pinpointed by the measured RQ (respiratory quotient, i.e. the ratio of CO2 production to O2 consumption) value of 1, as expected for fully aerobic glucose metabolism. Moreover, the sharp drop in the respiratory activity, confirmed that substrate was completely exhausted (Fig. 2). Indeed, over the first 30 h of incubation, the growth of the cell culture (measured as optical density) and utilisation of the substrate (indicated as CO2 production and O2 consumption) were closely associated. The biomass yield was calculated from the linear correlation between OD and CO2 emission, and O2 profiles. The net amount of biomass, corresponding to 1.2 OD units, was produced by consuming 1 g/L of glucose. Approximately one half of the initial amount of glucose was used as energy source, therefore oxidised by bacterial catabolic reactions, as by CO2 emission, accompanied by equimolar (~ 30 mM) oxygen consumption. It can be deduced that the remaining part of the organic carbon supplied was incorporated into the biomass by assimilation reactions. The exponential growth phase with RQ = 1 was followed by a peak of O2 consumption/CO2 production with RQ value of 0.8 accompanied by biomass (OD600) decrease. This indicated the respiration of intracellular storage substance(s).
In cultures grown at higher glucose concentrations, consumption of the substrate was observed even after reaching the peak of growth. In the sample cultured in 5 g/L glucose medium, the carbon substrate was totally consumed 2 days after the growth peak. In the 10 g/L glucose culture, 3 g/L of the carbon source were still detected after 7 days of growth (Fig. 1D). HPLC measurements did not reveal the production of secondary metabolites or organic acids.
Bioleaching of iron impurities from quartz sand
The ability of Acidiphilium sp. SJH cells to reduce ferric iron was assessed by adding 40% quartz sand to cultures grown in different media up to either the stationary or the exponential phase of growth. Moreover, different aeration conditions were applied as described in the material and methods section (Fig. 3).
Stationary phase cells yielded poor iron solubilization and the best performing culture was the one cultivated in galactose medium. Highest iron recovery was 6.6 ± 0.7 mg/L, 1.9 ± 0.2 mg/L and 4.1 ± 0.4 mg/L after 4 weeks of incubation with quartz sand under microaerobic, aerobic and full aeration conditions respectively (Fig. 3A and Table 2). During bioleaching, cell growth was not detected (data not reported), since quartz sand was added to cells already in the stationary phase and no other nutrients were supplemented.
In experiments performed with cells in the exponential phase of growth (Fig. 3B and Table 2), poor iron reduction was observed under full aeration conditions, whereas in microaerobic and aerobic conditions, cells grown in galactose media yielded highest iron recovery, respectively of 47 ± 2.4 mg/L and 46.8 ± 2.3 mg/L after 4 weeks of incubation with quartz sand (p-value < 0.01 in microaerobic and aerobic bioleaching condition between glucose, galactose, glycerol and minimal media, Tables S1 and S2). Fe2+ extraction occurred also in glucose and glycerol media, both in microaerobic and aerobic conditions, despite a lower yield. Doubling the biomass to sand ratio did not significantly increase iron solubilisation (data not shown). During exponential phase bioleaching, the cultures kept growing under all tested conditions (Fig. 4). Highest cell growth was detected under full aeration conditions (Fig. 4C), with 2.09 OD600 cell density achieved in glucose medium after 3 days of bioleaching. (p-value < 0.01 in full aerobic bioleaching condition between glucose, galactose, glycerol and minimal media, Table S3). In media supplemented with galactose or glycerol, the highest cell density was reached at the same time. Interestingly, the glucose-based culture maintained a constant density for over two weeks. In microaerobic and aerobic conditions, cell growth was reduced, although constant, over 4 weeks.
Since the light (heavy-minerals-free) fraction of quartz sand still contains iron impurities, in quantities that exceed the limits for the raw material for glass production (Table 1), in another series of experiments, the light product of gravity separation was subjected to bioleaching under the conditions previously defined as optimal (Fig. 5). In the presence of iron reducing bacteria most of the soluble iron was in divalent form. Total iron concentration in the leaching solutions reached approximately 55 mg/L after 4 weeks, which corresponded to a yield of 11% iron from the solid sample. The minute amounts of ferric iron in abiotic series likely resulted from dissolution of iron oxides in the raw material triggered by the acidic medium.
Analysis of cell components by whole cells by Fourier-transform infrared (FTIR) spectroscopy
Iron bioleaching did not affect the lipid compositions of cells since the marker signal at 1746 cm− 1 wavenumber underwent similar changes in the presence and in the absence of quartz sand (Fig. 6A). Likewise, the signals responsive to total proteins (1658 and 1630 cm− 1 wavenumbers) were unaltered (Fig. 6B). It was also noticed that the signal corresponding to the alpha-helical component of the proteins (1658 cm− 1 wavenumber) did not change, while the one given by the beta component of the total proteins (1630 cm− 1 wavenumber) decreased. This behaviour, however, was similarly found in the control sample, indicating that the variation was not induced by the bioleaching conditions. On this basis, it was possible to conclude that the presence of quartz sand and the activity of cells in iron bioleaching do not significantly affect major cell macromolecular components.
FTIR second derivative spectra of total lipid and total proteins of Acidiphilium sp. SJH whole cell. Total lipid signal at 1746 cm-1 wavenumber (carbonyl group, panel A) and total protein alpha-helix and beta-sheets signal, respectively at 1658 and 1630 cm-1 wavenumbers (B). CTR: Control condition, QS: Quartz Sand bioleaching condition
FTIR analysis highlighted the synchronised increase of peaks at specific wavenumbers, that is 980, 1057, 1100, 1132, 1187 and 1740 − 1720 cm− 1 (Figs. 7 and 8). The synchronous variation of these signals made it possible to assign them all to the same molecule, identified as poly-β-hydroxybutyrate (PHB) (Kansiz et al. 2000). FTIR analyses revealed the presence of PHB from the beginning of the bioleaching phase, suggesting that synthesis and accumulation of this polymer started during the biomass growth phase. Over time, the analyses monitored an increase of PHB after 7 and after 14 days of bioleaching. The synthesis and accumulation of PHB was verified not only under bioleaching conditions, but also in the cellular control in the absence of quartz sand, albeit at lower levels.
Intensity and variation over time of the peaks responsive to PHB recorded by FTIR. CTR: Control condition; QS: Quartz Sand bioleaching condition. E. coli signals were used as reference. *Peak Area; **Peak Intensity. CO: carbonylic group C = O (1740 − 1720 wavenumbers); AI: Amide I region (1700 − 1600 wavenumbers)
Discussion
The aim of this work was to gain insight into the physiology of Acidiphilium sp. SJH during bioleaching of iron impurities. To this end different conditions of growth (carbon source, aeration condition) were tested and the relationships between biomass growth and bioleaching performance evaluated. This acidophilic bacterium was shown to grow on all provided carbon sources, albeit with different duplication rates. Glucose was the carbon source most rapidly depleted and supported fastest microbial growth, in comparison to galactose and glycerol. As expected, increasing glucose concentrations (5 g/L and 10 g/L) resulted in higher biomass growth. However, while in media containing up to 5 g/L glucose the carbon source was consumed by the day the highest cell density was reached, when growing at 10 g/L glucose cells consumed only 7 g/L, ruling out the hypothesis that a further increase could help achieve higher culture density before bioleaching. While it is difficult to comment on the behaviour of Acidiphilium sp. SJH on glucose, due to the paucity of data reported in the literature, the ability of this strain to uptake and consume galactose has been reported (Johnson and McGinness 1991b; Johnson 1995). Galactose is a carbon and energy source present in acidic environments, such as acid mines, mainly in the form of extracellular polymeric substances (EPS) (More et al. 2014; Saavedra et al. 2020). These compounds, mainly formed by polysaccharides, proteins, lipids, and extracellular DNA (Zafra et al. 2012; Li et al. 2020b) are synthesised by the various organisms that inhabit these environments such as Ferroplasma acidiphilum (Hossein Karimi Darvanjooghi et al. 2023), Acidiphilium sp. C61 (Li et al. 2020b) or Acidithiobacillus ferrooxidans (Barreto et al. 2005), as a defence against environmental stress. A galactose transporter (GalP) was identified in Acidithiobacillus ferrooxidans, where galactose is taken up by the microorganism but is not involved in metabolic pathways associated with energy and biomass production (Barreto et al. 2005). In contrast, in Acidiphilium sp. C61, genomic analyses showed the presence of transporters for ribose, fructose and xylose but not specific transporters for galactose. In this scenario, the genome analyses of Acidiphilium sp. SJH would be useful to unveil the metabolic pathways of this bacterium.
Although Acidiphilium sp. SJH is an aerobic organism, highest iron bioleaching yields have been obtained under microaerobic and aerobic conditions, while only small amounts of iron were solubilized in conditions of full aeration. Oxygen has been reported to play a key role in the reduction of ferric iron by Acidiphilium bacteria (Lonergan et al. 1996). However, different results have been described for different strains. Acidiphilium rubrum and Acidiphilium acidophilum have been shown to reduce Fe3+ at low oxygen levels (Hallberg and Barrie Johnson 2001; Johnson and Bridge 2002), whereas the same process has been reported to be independent on oxygenation in Acidiphilium sp. SJH, Acidiphilium multivorum and Acidiphilium organovorum. However, the results of this work showed that the reduction of ferric iron by Acidiphilium sp. SJH does depend on aeration, in fact it has been obtained under microaerobic and aerobic conditions, whereas it is poorly observed under full aeration. This could probably be explained by high oxygen competing with ferric iron as the final acceptor of the electron transport chain.
In this study, the dissolution of about 5% of the ferric ion present in the quartz sand has been achieved. The yield is lower than that reported in other works performed with the same bacterial strain but with different starting materials. For example Kupka et al. (2007), used the magnetic fraction of quartz sand, which contains about 25% ferric iron vs. 0.22% of the quartz sand used in this research. In another work, the use of ferric iron in soluble form achieved similar iron reduction yields but in only few hours of incubation (Johnson and Bridge 2002). At the best of our knowledge, this is the first report about the performances of Acidiphilium sp. SJH to in the bioleaching of iron impurities on unprocessed raw material.
The harsh and stressful conditions of acidic environments, in which Acidiphilium spp. live, led to molecular adaptations, including both physiological modifications at the protein and lipid levels (Tatsuzawa et al. 1996; Mirete et al. 2017) and the production of molecules and polymers with anti-oxidant, anti-stress or protective function (Rani et al. 2021; Müller-Santos et al. 2021; More et al. 2014).
FTIR spectroscopy was used to highlight possible stress-related changes in cellular components. This analysis did not reveal any major changes in total lipids and proteins composition triggered by pH or bioleaching. PHB production was instead assessed in all samples and increased in those performing bioleaching. Thus, while the presence of quartz sand in the cultures did not trigger a general stress response at the level of macromolecular components, the higher content of the protective PHB polymer during bioleaching would suggest that some specific stress was indeed evoked (Müller-Santos et al. 2021). More specifically a toxic effect of the released ferrous iron may be hypothesised with consequent oxidative stress (Bennett and Gralnik 2019; Müller-Santos et al. 2021).
Some other Acidiphilium species, such as Acidiphilium sp. C61 (Li et al. 2020b), Acidiphilium sp. DX1-1 (Xu et al. 2013), Acidiphilium cryptum JF-5 (Küsel et al. 1999; Xu et al. 2013) and Acidiphilium sp. JA12-A1 (Ullrich et al. 2015) have been reported as capable of producing PHB. PHB producing bacteria can be divided into two groups (Lee 1996): the first one accumulates PHB during the stationary growth phase when nutrients are unbalanced, whereas the second group produces PHB during the growth regardless of nutrients ratio (Xu et al. 2013).
This is the first report of PHB production and accumulation by Acidiphilium sp. SJH, that can be included in the second group of PHB-producing bacteria. This feature, together with its ability to use various carbon sources, might support the introduction of Acidiphilium sp. SJH in a circular economy concept, where sugar-rich waste biomasses, such as molasses, might be the starting point of a parallel bioleaching & PHB production process.
Data availability
No datasets were generated or analysed during the current study.
References
Ami D, Duse A, Mereghetti P et al (2021) Tear-based vibrational spectroscopy applied to amyotrophic lateral sclerosis. Anal Chem 93:16995–17002. https://doi.org/10.1021/acs.analchem.1c02546
Ami D, Franco AR, Artusa V et al (2022) A global picture of molecular changes associated to LPS treatment in THP-1 derived human macrophages by Fourier transform infrared microspectroscopy. IJMS 23:13447. https://doi.org/10.3390/ijms232113447
Anjum F, Bhatti HN, Asgher M, Shahid M (2010) Leaching of metal ions from black shale by organic acids produced by Aspergillus Niger. Appl Clay Sci 47:356–361. https://doi.org/10.1016/j.clay.2009.11.052
Anjum F, Shahid M, Akcil A (2012) Biohydrometallurgy techniques of low grade ores: a review on black shale. Hydrometallurgy 117–118:1–12. https://doi.org/10.1016/j.hydromet.2012.01.007
Asghari I, Mousavi SM, Amiri F, Tavassoli S (2013) Bioleaching of spent refinery catalysts: a review. J Ind Eng Chem 19:1069–1081. https://doi.org/10.1016/j.jiec.2012.12.005
Baker-Austin C, Dopson M (2007) Life in acid: pH homeostasis in acidophiles. Trends Microbiol 15:165–171. https://doi.org/10.1016/j.tim.2007.02.005
Barreto M, Jedlicki E, Holmes DS (2005) Identification of a gene cluster for the formation of extracellular polysaccharide precursors in the chemolithoautotroph Acidithiobacillus ferrooxidans. Appl Environ Microbiol 71:2902–2909. https://doi.org/10.1128/AEM.71.6.2902-2909.2005
Bas AD (2021) Quartz bleaching by phosphoric acid: an investigation on the relationship between Fe rejection rate and colour response. Miner Eng 161:106739. https://doi.org/10.1016/j.mineng.2020.106739
Basaran A, Tuovinen O (1986) An ultraviolet spectrophotometric method for the determination of pyrite and ferrous ion oxidation by Thiobacillus ferrooxidans. Appl Microbiol Biotechnol 24. https://doi.org/10.1007/BF00257060
Bennet BD, Gralnik JA (2019) Mechanisms of toxicity by and resistance to ferrous iron in anaerobicsystems Free Radic Biol Med 140: 167–171. https://doi.org/10.1016/j.freeradbiomed.2019.06.027
Bosecker K (1997) Bioleaching: metal solubilization by microorganisms. FEMS Microbiol Rev 20:591–604. https://doi.org/10.1111/j.1574-6976.1997.tb00340.x
Bridge TAM, Johnson BD (2000) Reductive dissolution of ferric iron minerals by Acidiphilium SJH. Geomicrobiol J 17:193–206. https://doi.org/10.1080/01490450050121161
Brierley CL (2010) Biohydrometallurgical prospects. Hydrometallurgy 104:324–328. https://doi.org/10.1016/j.hydromet.2010.03.021
Clark DA, Norris PR (1996) Acidimicrobium ferrooxidans gen. nov., sp. nov.: mixed-culture ferrous iron oxidation with Sulfobacillus species. Microbiology 142:785–790. https://doi.org/10.1099/00221287-142-4-785
D’Hugues P, Spolaore P, Consortium B (2008) Biohydrometallurgy applied to exploitation of black shale resources: overview of Bioshale FP6 European project. Trans Nonferrous Met Soc China 18:1485–1490. https://doi.org/10.1016/S1003-6326(09)60030-3
De Divitiis M, Ami D, Pessina A et al (2023) Cheese-whey permeate improves the fitness of Escherichia coli cells during recombinant protein production. Biotechnol Biofuels 16:30. https://doi.org/10.1186/s13068-023-02281-8
Doglia SM, Ami D, Natalello A et al (2008) Fourier transform infrared spectroscopy analysis of the conformational quality of recombinant proteins within inclusion bodies. Biotechnol J 3:193–201. https://doi.org/10.1002/biot.200700238
Du F, Li J, Li X, Zhang Z (2011) Improvement of iron removal from silica sand using ultrasound-assisted oxalic acid. Ultrason Sonochem 18:389–393. https://doi.org/10.1016/j.ultsonch.2010.07.006
Hallberg KB, Barrie Johnson D (2001) Biodiversity of acidophilic prokaryotes. Advances in Applied Microbiology. Elsevier, pp 37–84
Harrison AP (1981) Acidiphilium cryptum gen. nov., sp. nov., heterotrophic bacterium from acidic mineral environments. Int J Syst Bacteriol 31:327–332. https://doi.org/10.1099/00207713-31-3-327
Herrera L, Ruiz P, Aguillon JC, Fehrmann A (2007) A new spectrophotometric method for the determination of ferrous iron in the presence of ferric iron. J Chem Technol Biotechnol 44:171–181. https://doi.org/10.1002/jctb.280440302
Hossein Karimi Darvanjooghi M, Kaur K, Magdouli S, Kaur Brar S (2023) Extracellular polymeric substances overproduction strategy in Ferroplasma acidiphilum growth for biooxidation of low-grade gold bearing ore: role of monosaccharides. Bioresour Technol 369:128476. https://doi.org/10.1016/j.biortech.2022.128476
Johnson DB (1995) Selective solid media for isolating and enumerating acidophilic bacteria. J Microbiol Methods 23:205–218. https://doi.org/10.1016/0167-7012(95)00015-D
Johnson DB, Bridge TAM (2002) Reduction of ferric iron by acidophilic heterotrophic bacteria: evidence for constitutive and inducible enzyme systems in Acidiphilium spp. J Appl Microbiol 92:315–321. https://doi.org/10.1046/j.1365-2672.2002.01535.x
Johnson DB, McGinness S (1991a) Ferric iron reduction by acidophilic heterotrophic bacteria. Appl Environ Microbiol 57:207–211. https://doi.org/10.1128/aem.57.1.207-211.1991
Johnson DB, McGinness S (1991b) A highly efficient and universal solid medium for growing mesophilic and moderately thermophilic, iron-oxidizing, acidophilic bacteria. J Microbiol Methods 13:113–122. https://doi.org/10.1016/0167-7012(91)90011-E
Johnson DB, Kelso WI, Jenkins DA (1979) Bacterial streamer growth in a disused pyrite mine. Environmental Pollution (1970) 18:107–118. https://doi.org/10.1016/0013-9327(79)90086-7
Kansiz M, Billman-Jacobe H, McNaughton D (2000) Quantitative determination of the biodegradable polymer poly(β-hydroxybutyrate) in a recombinant Escherichia coli strain by use of mid-infrared spectroscopy and multivariative statistics. Appl Environ Microbiol 66:3415–3420. https://doi.org/10.1128/AEM.66.8.3415-3420.2000
Kelly DP, Wood AP (2000) Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov. and Thermithiobacillus gen. nov. International Journal of Systematic and Evolutionary Microbiology 50:511–516. https://doi.org/10.1099/00207713-50-2-511
Kupka D, Lovás M, Šepelák V (2007) Deferrization of kaolinic sand by iron oxidizing and iron reducing bacteria. AMR 20–21:130–133. https://doi.org/10.4028/www.scientific.net/AMR.20-21.130
Küsel K, Dorsch T, Acker G, Stackebrandt E (1999) Microbial Reduction of Fe(III) in acidic sediments: isolation of Acidiphilium cryptum JF-5 capable of coupling the reduction of Fe(III) to the oxidation of glucose. Appl Environ Microbiol 65:3633–3640. https://doi.org/10.1128/AEM.65.8.3633-3640.1999
Lee SY (1996) Plastic bacteria? Progress and prospects for polyhydroxyalkanoate production in bacteria. Trends Biotechnol 14:431–438
Li L, Liu Z, Zhang M et al (2020a) Insights into the metabolism and evolution of the genus Acidiphilium, a typical acidophile in acid mine drainage. mSystems 5:e00867–e00820. https://doi.org/10.1128/mSystems.00867-20
Li Q, Cooper RE, Wegner C-E, Küsel K (2020b) Molecular mechanisms underpinning aggregation in Acidiphilium sp. C61 isolated from iron-rich pelagic aggregates. Microorganisms 8:314. https://doi.org/10.3390/microorganisms8030314
Lonergan DJ, Jenter HL, Coates JD et al (1996) Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J Bacteriol 178:2402–2408. https://doi.org/10.1128/jb.178.8.2402-2408.1996
Malik L, Hedrich S (2022) Ferric iron reduction in extreme acidophiles. Front Microbiol 12:818414. https://doi.org/10.3389/fmicb.2021.818414
Mirete S, Morgante V, González-Pastor JE (2017) Acidophiles: diversity and mechanisms of adaptation to acidic environments. In: Stan-Lotter H, Fendrihan S (eds) Adaption of Microbial Life to Environmental extremes. Springer International Publishing, Cham, pp 227–251
More TT, Yadav JSS, Yan S et al (2014) Extracellular polymeric substances of bacteria and their potential environmental applications. J Environ Manage 144:1–25. https://doi.org/10.1016/j.jenvman.2014.05.010
Müller-Santos M, Koskimäki JJ, Alves LPS et al (2021) The protective role of PHB and its degradation products against stress situations in bacteria. FEMS Microbiol Rev 45:fuaa058. https://doi.org/10.1093/femsre/fuaa058
Norris PR, Burton NP, Foulis NAM (2000) Acidophiles in bioreactor mineral processing. Extremophiles 4:0071–0076. https://doi.org/10.1007/s007920050139
Ohmura N, Sasaki K, Matsumoto N, Saiki H (2002) Anaerobic respiration using Fe 3+, S 0, and H 2 in the chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans. J Bacteriol 184:2081–2087. https://doi.org/10.1128/JB.184.8.2081-2087.2002
Panda S, Akcil A, Pradhan N, Deveci H (2015a) Current scenario of chalcopyrite bioleaching: a review on the recent advances to its heap-leach technology. Bioresour Technol 196:694–706. https://doi.org/10.1016/j.biortech.2015.08.064
Panda S, Mishra S, Rao DS et al (2015b) Extraction of copper from copper slag: mineralogical insights, physical beneficiation and bioleaching studies. Korean J Chem Eng 32:667–676. https://doi.org/10.1007/s11814-014-0298-6
Platias S, Vatalis KI, Charalampides G (2014) Suitability of quartz sands for different industrial applications. Procedia Econ Finance 14:491–498. https://doi.org/10.1016/S2212-5671(14)00738-2
Rani A, Saini K, Bast F et al (2021) Microorganisms: a potential source of bioactive molecules for antioxidant applications. Molecules 26:1142. https://doi.org/10.3390/molecules26041142
Rohwerder T, Gehrke T, Kinzler K, Sand W (2003) Bioleaching review part A. Appl Microbiol Biotechnol 63:239–248. https://doi.org/10.1007/s00253-003-1448-7
Rothschild LJ, Mancinelli RL (2001) Life in extreme environments. Nature 409:1092–1101. https://doi.org/10.1038/35059215
Saavedra A, Aguirre P, Gentina JC (2020) Biooxidation of iron by Acidithiobacillus ferrooxidans in the presence of d-galactose: understanding its influence on the production of EPS and cell tolerance to high concentrations of iron. Front Microbiol 11:759. https://doi.org/10.3389/fmicb.2020.00759
Sajjad W, Zheng G, Din G et al (2019) Metals extraction from sulfide ores with microorganisms: the bioleaching technology and recent developments. Trans Indian Inst Met 72:559–579. https://doi.org/10.1007/s12666-018-1516-4
Srichandan H, Mohapatra RK, Parhi PK, Mishra S (2019) Bioleaching approach for extraction of metal values from secondary solid wastes: a critical review. Hydrometallurgy 189:105122. https://doi.org/10.1016/j.hydromet.2019.105122
Tatsuzawa H, Takizawa E, Wada M, Yamamoto Y (1996) Fatty acid and lipid composition of the acidophilic green alga Chlamydomonas sp. 1. J Phycol 32:598–601. https://doi.org/10.1111/j.0022-3646.1996.00598.x
Ullrich SR, Poehlein A, Voget S et al (2015) Permanent draft genome sequence of Acidiphilium sp. JA12-A1. Stand Genomic Sci 10:56. https://doi.org/10.1186/s40793-015-0040-y
Vašková A, Kupka D (2007) Reduction of soluble and solid ferric iron by Acidiphilium SJH. AMR 20–21:497–500. https://doi.org/10.4028/www.scientific.net/AMR.20-21.497
Veglio’ F (1997) Factorial experiments in the development of a kaolin bleaching process using thiourea in sulphuric acid solutions. Hydrometallurgy 45:181–197. https://doi.org/10.1016/S0304-386X(96)00078-3
Wang X, Ma L, Wu J et al (2020) Effective bioleaching of low-grade copper ores: insights from microbial cross experiments. Bioresour Technol 308:123273. https://doi.org/10.1016/j.biortech.2020.123273
Xu A, Xia J, Song Z et al (2013) The effect of energy substrates on PHB accumulation of Acidiphilium cryptum DX1-1. Curr Microbiol 67:379–387. https://doi.org/10.1007/s00284-013-0373-y
Yue G, Guezennec A-G, Asselin E (2016) Extended validation of an expression to predict ORP and iron chemistry: application to complex solutions generated during the acidic leaching or bioleaching of printed circuit boards. Hydrometallurgy 164:334–342. https://doi.org/10.1016/j.hydromet.2016.06.027
Zafra O, Lamprecht-Grandío M, De Figueras CG, González-Pastor JE (2012) Extracellular DNA release by undomesticated Bacillus subtilis is regulated by early competence. PLoS ONE 7:e48716. https://doi.org/10.1371/journal.pone.0048716
Zhang Z, Li J, Li X et al (2012) High efficiency iron removal from quartz sand using phosphoric acid. Int J Miner Process 114–117:30–34. https://doi.org/10.1016/j.minpro.2012.09.001
Acknowledgements
We thank Antonino Natalello and Diletta Ami from the University of Milano-Bicocca for performing IR measurements.
Funding
Open access funding provided by Università degli Studi di Milano - Bicocca within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
D.K., P.B. and M.L. planned and supervised the work, wrote and reviewed the manuscript. A.M.,V.S., Z.B., L.H. and S.H. performed experimental work and analysis or the results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Yusuf Akhter.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
*Corresponding Author:
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marchetti, A., Kupka, D., Senatore, V.G. et al. Iron bioleaching and polymers accumulation by an extreme acidophilic bacterium. Arch Microbiol 206, 275 (2024). https://doi.org/10.1007/s00203-024-04005-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-024-04005-4