Abstract
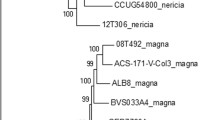
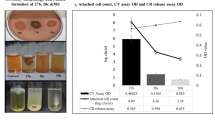
Acinetobacter species encode for extracellularly secreted Biofilm-associated protein (Bap), a multi-domain protein with variable molecular weights reaching several hundred kilodaltons. Bap is crucial for the development of multi-dimensional structures of mature biofilms. In our investigation, we analyzed 7338 sequences of A. baumannii from the NCBI database and found that Bap or Bap-like protein (BLP) was present in 6422 (87.52%) isolates. Further classification revealed that 12.12% carried Type-1 Bap, 68.44% had Type-2, 6.91% had Type-3, 0.05% had Type-6 or SDF-Type, and 12.51% lacked Bap or BLP. The majority of isolates with Type-1, Type-2, and Type-3 Bap belonged to ST1, ST2, and ST25, respectively. Phylogenetic analysis suggested that Type-1 Bap is the most ancient, while Type-3 and SDF-Type have evolved recently. Studying the interaction of predicted Bap structures with human CEACAM-1 and PIgR showed that Bap with its BIg13 and BIg6 domains interact with the N-terminal domain of CEACAM-1, involving Arg43 and Glu40, involved in CEACAM-1 dimerization. Also, we found that recently evolved Type-3 and SDF-Type Bap showed greater interaction with CEACAM-1 and PIgR. It can be asserted that the evolution of Bap has conferred enhanced virulence characteristics to A. baumannii with increased interaction with CEACAM-1 and PIgR. Using in silico approaches, this study explores the evolutionary, physicochemical, and structural features of A. baumannii Bap and unravels its crucial role in mediating interaction with human CEACAM-1 and PIgR through detailed structure modelling. These findings advance our understanding of A. baumannii Bap and highlight its role in pathogenesis.






Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Agarwal V, Asmat TM, Dierdorf NI, Hauck CR, Hammerschmidt S (2010) Polymeric immunoglobulin receptor-mediated invasion of Streptococcus pneumoniae into host cells requires a coordinate signaling of SRC family of protein-tyrosine kinases, ERK, and c-Jun N-terminal kinase. J Biol Chem 285(46):35615–35623. https://doi.org/10.1074/jbc.M110.172999
Alsan M, Klompas M (2010) Acinetobacter baumannii: an emerging and important pathogen. J Clin Outcomes Management: JCOM 17(8):363
Ambrosi C, Scribano D, Sarshar M, Zagaglia C, Singer BB, Palamara AT (2020) Acinetobacter baumannii targets human carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) for invasion of pneumocytes. Msystems 5(6):10–1128. https://doi.org/10.1128/msystems.00604-20
Bamberger AM, Sudahl S, Löning T, Wagener C, Bamberger CM, Drakakis P, Coutifaris C, Makrigiannakis A (2000) The adhesion molecule CEACAM1 (CD66a, C-CAM, BGP) is specifically expressed by the extravillous intermediate trophoblast. Am J Pathol 156(4):1165–1170. https://doi.org/10.1016/S0002-9440(10)64985-1
Baranov V, Hammarström S (2004) Carcinoembryonic antigen (CEA) and CEA-related cell adhesion molecule 1 (CEACAM1), apically expressed on human colonic M cells, are potential receptors for microbial adhesion. Histochem Cell Biol 121:83–89. https://doi.org/10.1007/s00418-003-0613-5
Bowie JU, Lüthy R, Eisenberg D (1991) A method to identify protein sequences that fold into a known three-dimensional structure. Science 253(5016):164–170. https://doi.org/10.1126/science.1853201
Brock SC, McGraw PA, Wright PF, Crowe JE Jr (2002) The human polymeric immunoglobulin receptor facilitates invasion of epithelial cells by Streptococcus pneumoniae in a strain-specific and cell type-specific manner. Infect Immun 70(9):5091–5095. https://doi.org/10.1128/iai.70.9.5091-5095.2002
Brossard KA, Campagnari AA (2012) The Acinetobacter baumannii biofilm-associated protein plays a role in adherence to human epithelial cells. Infect Immun 80(1):228–233. https://doi.org/10.1128/iai.05913-11
Catton EA, Bonsor DA, Herrera C, Stålhammar-Carlemalm M, Lyndin M, Turner CE, McCarthy AJ (2023) Human CEACAM1 is targeted by a Streptococcus pyogenes adhesin implicated in puerperal sepsis pathogenesis. Nat Commun 14(1):2275. https://doi.org/10.1038/s41467-023-37732-1
Chapartegui-González I, Lázaro-Díez M, Bravo Z, Navas J, Icardo JM, Ramos-Vivas J (2018) Acinetobacter baumannii maintains its virulence after long-time starvation. PLoS ONE, 13(8), e0201961
Chatterjee S, Basak AJ, Nair AV, Duraivelan K, Samanta D (2021) Immunoglobulin-fold containing bacterial adhesins: molecular and structural perspectives in host tissue colonization and infection. FEMS Microbiol Lett 368(2):fnaa220. https://doi.org/10.1093/femsle/fnaa220
Chen CL, Dudek A, Liang YH, Janapatla RP, Lee HY, Hsu L, Kuo HY, Chiu CH (2022) d-mannose-sensitive pilus of Acinetobacter baumannii is linked to biofilm formation and adherence onto respiratory tract epithelial cells. J Microbiol Immunol Infect 55(1):69–79. https://doi.org/10.1016/j.jmii.2021.01.008
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2(9):1511–1519. https://doi.org/10.1002/pro.5560020916
De Breij A, Eveillard M, Dijkshoorn L, Van Den Broek PJ, Nibbering PH, Joly-Guillou ML (2012) Differences in Acinetobacter baumannii strains and host innate immune response determine morbidity and mortality in experimental pneumonia. PLoS ONE 7(2):e30673. https://doi.org/10.1371/journal.pone.0030673
De Gregorio E, Roscetto E, Iula VD, Martinucci M, Zarrilli R, Di Nocera PP, Catania MR (2015) Development of a real-time PCR assay for the rapid detection of Acinetobacter baumannii from whole blood samples. New Microbiol 38(2):251–257
Eze EC, Chenia HY, El Zowalaty ME (2018) Acinetobacter baumannii biofilms: effects of physicochemical factors, virulence, antibiotic resistance determinants, gene regulation, and future antimicrobial treatments. Infection and drug resistance, pp 2277–2299
Gasteiger E, Hoogland C, Gattiker A, Duvaud SE, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server (pp. 571–607). Humana press. https://doi.org/10.1385/1-59259-890-0:571
Gedefie A, Demsis W, Ashagrie M, Kassa Y, Tesfaye M, Tilahun M, Bisetegn H, Sahle Z (2021) Acinetobacter baumannii biofilm formation and its role in disease pathogenesis: a review. Infect Drug Resist 3711–3719. https://doi.org/10.2147/IDR.S332051
Giannouli M, Antunes LC, Marchetti V, Triassi M, Visca P, Zarrilli R (2013) Virulence-related traits of epidemic Acinetobacter baumannii strains belonging to the international clonal lineages I-III and to the emerging genotypes ST25 and ST78. BMC Infect Dis 13:1–11. https://doi.org/10.1186/1471-2334-13-282
Goh HS, Beatson SA, Totsika M, Moriel DG, Phan MD, Szubert J, Runnegar N, Sidjabat HE, Paterson DL, Nimmo GR, Lipman J (2013) Molecular analysis of the Acinetobacter baumannii biofilm-associated protein. Appl Environ Microbiol 79(21):6535–6543. https://doi.org/10.1128/AEM.01402-13
Greene C, Vadlamudi G, Newton D, Foxman B, Xi C (2016a) The influence of biofilm formation and multidrug resistance on environmental survival of clinical and environmental isolates of Acinetobacter baumannii. Am J Infect Control 44(5):e65–e71. https://doi.org/10.1016/j.ajic.2015.12.012
Greene C, Wu J, Rickard AH, Xi C (2016b) Evaluation of the ability of Acinetobacter baumannii to form biofilms on six different biomedical relevant surfaces. Lett Appl Microbiol 63(4):233–239. https://doi.org/10.1111/lam.12627
Hammarström S (1999) The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. In Seminars in cancer biology (Vol. 9, No. 2, pp. 67–81). Academic Press. https://doi.org/10.1006/scbi.1998.0119
Hill DJ, Edwards AM, Rowe HA, Virji M (2005) Carcinoembryonic antigen-related cell adhesion molecule (CEACAM)‐binding recombinant polypeptide confers protection against infection by respiratory and urogenital pathogens. Mol Microbiol 55(5):1515–1527. https://doi.org/10.1111/j.1365-2958.2005.04487.x
Howard A, O’Donoghue M, Feeney A, Sleator RD (2012) Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence 3(3):243–250. https://doi.org/10.4161/viru.19700
Hu Y, He L, Tao X, Meng F, Zhang J (2016) Biofilm may not be necessary for the Epidemic Spread of Acinetobacter baumannii. Sci Rep 6(1):32066. https://doi.org/10.1038/srep32066
Jung SY, Lee SH, Lee SY, Yang S, Noh H, Chung EK, Lee JI (2017) Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: a systemic review and bayesian network meta-analysis. Crit Care 21(1):1–15. https://doi.org/10.1186/s13054-017-1916-6
Kahya HF, Andrew PW, Yesilkaya H (2017) Deacetylation of sialic acid by esterasespotentiates pneumococcal neuraminidase activity for mucin utilization, colonization andvirulence. PLoS Pathog 13, e1006263
Kale SD, Dikshit N, Kumar P, Balamuralidhar V, Khameneh HJ, Bin Abdul Malik N, Koh TH, Tan GGY, Tan TT, Mortellaro A, Sukumaran B (2017) Nod2 is required for the early innate immune clearance of Acinetobacter baumannii from the lungs. Sci Rep 7(1):17429. https://doi.org/10.1038/s41598-017-17653-y
Königer V, Holsten L, Harrison U, Busch B, Loell E, Zhao Q, Bonsor DA, Roth A, Kengmo-Tchoupa A, Smith SI, Mueller S, Haas R (2016) Helicobacter pylori exploits human CEACAMs via HopQ for adherence and translocation of CagA. Nat Microbiol 2(1):1–12. https://doi.org/10.1038/nmicrobiol.2016.188
Kuespert K, Roth A, Hauck CR (2011) Neisseria meningitidis has two independent modes of recognizing its human receptor CEACAM1. PLoS ONE 6(1):e14609. https://doi.org/10.1371/journal.pone.0014609
Laskowski RA, MacArthur MW, Thornton JM (1998) Validation of protein models derived from experiment. Curr Opin Struct Biol 8(5):631–639. https://doi.org/10.1016/S0959-440X(98)80156-5
Lázaro-Díez M, Navascués-Lejarza T, Remuzgo-Martínez S, Navas J, Icardo JM, Acosta F, Ramos-Vivas J (2016) Acinetobacter baumannii and A. pittii clinical isolates lack adherence and cytotoxicity to lung epithelial cells in vitro. Microbes Infect 18(9):559–564
Loehfelm TW, Luke NR, Campagnari AA (2008) Identification and characterization of an Acinetobacter baumannii biofilm-associated protein. J Bacteriol 190(3):1036–1044. https://doi.org/10.1128/jb.01416-07
Lüthy R, Bowie JU, Eisenberg D (1992) Assessment of protein models with three-dimensional profiles. Nature 356(6364):83–85. https://doi.org/10.1038/356083a0
Maure A, Robino E, Van der Henst C (2023) The intracellular life of Acinetobacter baumannii. Trends Microbiol. https://doi.org/10.1016/j.tim.2023.06.007
Mix AK, Goob G, Sontowski E, Hauck CR (2021) Microscale communication between bacterial pathogens and the host epithelium. Genes Immun 22(5–6):247–254. https://doi.org/10.1038/s41435-021-00149-1
Morris FC, Dexter C, Kostoulias X, Uddin MI, Peleg AY (2019) The mechanisms of disease caused by Acinetobacter baumannii. Front Microbiol 10:1601. https://doi.org/10.3389/fmicb.2019.01601
Najjar SM (2002) Regulation of insulin action by CEACAM1. Trends Endocrinol Metabolism 13(6):240–245. https://doi.org/10.1016/S1043-2760(02)00608-2
Öbrink B (1997) CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol 9(5):616–626. https://doi.org/10.1016/S0955-0674(97)80114-7
Rangel A, Steenbergen SM, Vimr ER (2016) Unexpected diversity of Escherichia coli sialate O-acetyl esterase NanS. J Bacteriol 198(20):2803–2809. https://doi.org/10.1128/jb.00189-16
Ribet D, Cossart P (2010) Pathogen-mediated posttranslational modifications: a re-emerging field. Cell 143(5):694–702
Sheikh A, Fleckenstein JM (2023) Interactions of pathogenic Escherichia coli with CEACAMs. Front Immunol 14:1120331. https://doi.org/10.3389/fimmu.2023.1120331
Singer BB, Scheffrahn I, Heymann R, Sigmundsson K, Kammerer R, Öbrink B (2002) Carcinoembryonic antigen-related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol-linked proteins in human leukocytes. J Immunol 168(10):5139–5146. https://doi.org/10.4049/jimmunol.168.10.5139
van Sorge NM, Bonsor DA, Deng L, Lindahl E, Schmitt V, Lyndin M, Schmidt A, Nilsson OR, Brizuela J, Boero E, Sundberg EJ (2021) Bacterial protein domains with a novel Ig-like Fold target human CEACAM receptors. EMBO J 40(7):106103. https://doi.org/10.15252/embj.2020106103
Viale AM, Evans BA (2020) Microevolution in the major outer membrane protein OmpA of Acinetobacter baumannii. Microb Genomics 6(6). https://doi.org/10.1099/mgen.0.000381
Villullas S, Hill DJ, Sessions RB, Rea J, Virji M (2007) Mutational analysis of human CEACAM1: the potential of receptor polymorphism in increasing host susceptibility to bacterial infection. Cell Microbiol 9(2):329–346. https://doi.org/10.1111/j.1462-5822.2006.00789.x
Wollacott AM, Zanghellini A, Murphy P, Baker D (2007) Prediction of structures of multidomain proteins from structures of the individual domains. Protein Sci 16(2):165–175. http://www.proteinscience.org/cgi/doi/10.1110/ps.062270707
Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B (2017) Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 30(1):409–447. https://doi.org/10.1111/j.1462-5822.2006.00789.x
Yousef F, Espinosa-Urgel M (2007) Silico analysis of large microbial surface proteins. Res Microbiol 158(6):545–550. https://doi.org/10.1016/j.resmic.2007.04.006
Zilberberg MD, Kollef MH, Shorr AF (2016) Secular trends in Acinetobacter baumannii resistance in respiratory and blood stream specimens in the United States, 2003 to 2012: a survey study. J Hosp Med 11(1):21–26. https://doi.org/10.1002/jhm.2477
Acknowledgements
This work was supported by ICMR-National Institute of Pathology, New Delhi, India. KU acknowledges the University Grant Commission (UGC), New Delhi, India, for providing fellowship.
Funding
ICMR-National Institute of Pathology.
Author information
Authors and Affiliations
Contributions
KU, RK, QMR, and RS conceived and designed the study. KU and RK performed the experiments. KU, RK, and RS analyzed the data. KU RK and RS drafted the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Yusuf Akhter.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Upmanyu, K., Kumar, R., Rizwanul Haque, Q.M. et al. Exploring the evolutionary and pathogenic role of Acinetobacter baumannii biofilm-associated protein (Bap) through in silico structural modeling. Arch Microbiol 206, 267 (2024). https://doi.org/10.1007/s00203-024-03992-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-024-03992-8