Abstract
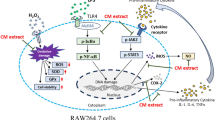
Crocodiles are renowned for their resilience and capacity to withstand environmental stressors, likely influenced by their unique gut microbiome. In this study, we determined whether selected gut bacteria of Crocodylus porosus exhibit anti-inflammatory effects in response to stress, by measuring nitric oxide release, interleukin 1-beta, tumor necrosis factor-alpha, and prostaglandin E2 in cerebrovascular endothelial cells. Using the Griess assay, the findings revealed that among several C. porosus gut bacterial isolates, the conditioned media containing the metabolites of two bacterial strains (CP27 and CP36) inhibited nitric oxide production significantly, in response to the positive control, i.e., taxol-treatment. Notably, CP27 and CP36 were more potent at reducing nitric oxide production than senloytic compounds (fisetin, quercetin). Using enzyme linked immunosorbent assays, the production of pro-inflammatory cytokines (IL-1β, TNF-α, PGE2), was markedly reduced by treatment with CP27 and CP36, in response to stress. Both CP27 and CP36 contain a plethora of metabolites to exact their effects [(3,4-dihydroxyphenylglycol, 5-methoxytryptophan, nifedipine, 4-chlorotestosterone-17-acetate, 3-phenoxypropionic acid, lactic acid, f-Honaucin A, l,l-Cyclo(leucylprolyl), 3-hydroxy-decanoic acid etc.], indicative of their potential in providing protection against cellular stress. Further high-throughput bioassay-guided testing of gut microbial metabolites from crocodiles, individually as well as in combination, together with the underlying molecular mechanisms, in vitro and in vivo will elucidate their value in the rational development of innovative therapies against cellular stress/gut dysbiosis.









Similar content being viewed by others
Availability of data and materials
The data is available upon request to the corresponding author.
References
Al-Alami O, Sammons J, Martin JH, Hassan HT (1998) Divergent effect of taxol on proliferation, apoptosis and nitric oxide production in MHH225 CD34 positive and U937 CD34 negative human leukaemia cells. Leuk Res 22(10):939–945
Arcidiacono S, Soares JW, Philip Karl J, Chrisey L, Dancy CPT, Goodson M, Gregory F, Hammamieh R, Loughnane NK, Kokoska R, Riddle CAPT (2018) The current state and future direction of DoD gut microbiome research: a summary of the first DoD gut microbiome informational meeting. Stand in Genomic Sci 13:5
Behl T, Rana T, Sehgal A, Makeen HA, Albratty M, Alhazmi HA, Meraya AM, Bhatia S, Sachdeva M (2023) Phytochemicals targeting nitric oxide signaling in neurodegenerative diseases. Nitric Oxide 130:1–11
Bryan NS, Grisham MB (2007) Methods to detect nitric oxide and its metabolites in biological samples. Free Rad Biol Med 43(5):645–657
Canossa M, Giordano E, Cappello S, Guarnieri C, Ferri S (2002) Nitric oxide down-regulates brain-derived neurotrophic factor secretion in cultured hippocampal neurons. Proc Nat Acad Sci 99(5):3282–3287
Chaib S, Tchkonia T, Kirkland JL (2022) Cellular senescence and senolytics: the path to the clinic. Nat Med 28(8):1556–1568
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13(10):701–712
Esch T, Stefano GB, Fricchione GL, Benson H (2002) Stress-related diseases-a potential role for nitric oxide. Med Sci Monit 8(6):103–118
Golovatscka V, Ennes H, Mayer EA, Bradesi S (2012) Chronic stress-induced changes in pro-inflammatory cytokines and spinal glia markers in the rat: a time course study. Neuroimmunomod 19(6):367–376
Grebenchtchikov N, van der Ven-Jongekrijg J, Pesman GJ, Geurts-Moespot A, van der Meer JW, Sweep FC (2005) Development of a sensitive ELISA for the quantification of human tumour necrosis factor-α using 4 polyclonal antibodies. Eur Cyto Net 16(3):215–222
Han Y, Kim SY (2023) Endothelial senescence in vascular diseases: current understanding and future opportunities in senotherapeutics. Exp Mol Med 55(1):1–12
Janke A, Gullberg A, Hughes S, Aggarwal RK, Arnason U (2005) Mitogenomic analyses place the gharial (Gavialis gangeticus) on the crocodile tree and provide pre-K/T divergence times for most crocodilians. J Mol Evo 61:620–626
Ke J, Yang Y, Che Q, Jiang F, Wang H, Chen Z, Zhu M, Tong H, Zhang H, Yan X, Wang X (2016) Prostaglandin E2 (PGE2) promotes proliferation and invasion by enhancing SUMO-1 activity via EP4 receptor in endometrial cancer. Tumor Biol 37(9):12203–12211
Khan NA, Soopramanien M, Maciver SK, Anuar TS, Sagathevan K, Siddiqui R (2021) Crocodylus porosus gut bacteria: a possible source of novel metabolites. Molecules 26(16):e4999
Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J (2001) Transient expression of IL-1β induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest 107(12):1529–1536
Laissue JA, Chappuis B, Müller C, Reubi JC, Gebbers JO (1993) The intestinal immune system and its relation to disease. Dig Dis 11(4–5):298–312
Lamaudière MT, Arasaradnam R, Weedall GD, Morozov IY (2023) The colorectal cancer microbiota alter their transcriptome to adapt to the acidity, reactive oxygen species, and metabolite availability of gut microenvironments. Msphere 27:e00627-e722
Leeming ER, Johnson AJ, Spector TD, Le Roy CI (2019) Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients 11(12):2862
Lyte JM, Gheorghe CE, Goodson MS, Kelley-Loughnane N, Dinan TG, Cryan JF, Clarke G (2020) Gut-brain axis serotonergic responses to acute stress exposure are microbiome-dependent. Neurogastroenterol Motil 32(11):e13881
Moens E, Veldhoen M (2012) Epithelial barrier biology: good fences make good neighbours. Immunol 135(1):1–8
Nathan C (1992) Nitric oxide as a secretory product of mammalian cells. FASEB J 6(12):3051–3064
Pagliaro P (2003) Differential biological effects of products of nitric oxide (NO) synthase: it is not enough to say NO. Life Sci 73(17):2137–2149
Sarkar A, Karmakar S, Bhattacharyya S, Purkait K, Mukherjee A (2015) Nitric oxide release by N-(2-chloroethyl)-N-nitrosoureas: a rarely discussed mechanistic path towards their anticancer activity. RSC Adv 5(3):2137–2146
Siddiqui R, Khan NA (2023) Microbiome and one health: potential of novel metabolites from the gut microbiome of unique species for human health. Microorganisms 11(2):481
Siddiqui R, Maciver S, Elmoselhi A, Soares NC, Khan NA (2021) Longevity, cellular senescence and the gut microbiome: lessons to be learned from crocodiles. Heliyon 7(12):e08594
Siddiqui R, Maciver SK, Khan NA (2022) Gut microbiome–immune system interaction in reptiles. J Appl Microbiol 132(4):2558–2571
Siddiqui R, Akbar N, Soares NC, Al-Hroub HM, Semreen MH, Maciver SK, Khan NA (2023) Mass spectrometric analysis of bioactive conditioned media of bacteria isolated from reptilian gut. Future Sci OA 9(5):FSO861
Stockdale MT, Benton MJ (2021) Environmental drivers of body size evolution in crocodile-line archosaurs. Comm Biol 4(1):38
Tse JK (2017) Gut microbiota, nitric oxide, and microglia as prerequisites for neurodegenerative disorders. ACS Chem Neurosci 8(7):1438–1447
Vieira LM, Nunes VDS, Amaral MDA, Oliveira AC, Hauser-Davis RA, Campos RC (2011) Mercury and methyl mercury ratios in caimans (Caiman crocodilus yacare) from the Pantanal area. Brazil J Environ Monit 13(2):280–287
Willson NL, Van TT, Lever J, Moore RJ, Stanley D (2019) Characterisation of the intestinal microbiota of commercially farmed saltwater crocodiles, Crocodylus porosus. Appl Microbiol Biotechnol 103:8977–8985
Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, Niedernhofer LJ, Robbins PD, Tchkonia T, Kirkland JL (2017) New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging 9(3):955
Funding
This work was funded by the Air Force Office of Scientific Research (AFOSR), grant number: FA 8655-20-1-7004.
Author information
Authors and Affiliations
Contributions
RS and NAK conceptualized the study amid discussions with SKM. NA and RS carried out all experimental work amid critical discussions with NAK, AMA, HF, and SKM. RS prepared the first draft, while SKM, NA and NAK corrected and finalized the manuscript. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethical approval
No animals or humans were used in this study.
Additional information
Communicated by Ran Wang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siddiqui, R., Akbar, N., Maciver, S.K. et al. Gut microbiome of Crocodylus porosus and cellular stress: inhibition of nitric oxide, interleukin 1-beta, tumor necrosis factor-alpha, and prostaglandin E2 in cerebrovascular endothelial cells. Arch Microbiol 205, 344 (2023). https://doi.org/10.1007/s00203-023-03680-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-023-03680-z