Abstract
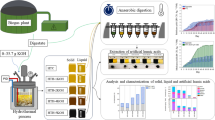
In this study, heat-pretreated sulfate-reducing bacteria (SRBs) were evaluated for simultaneous sulfate and nitrate removal in a bioelectrochemical system (BES). The effect of the applied potential of 20 mV to SRBs was evaluated at a sulfate concentration of 3 g/L and/or nitrate concentration of 0.5 g/L supplemented before heat pretreatment for sulfate and nitrate removal. The highest H2 production of 2.24 ± 0.04 mM/L in heat-pretreated culture was observed in the presence of sulfate at an applied potential of 20 mV (BHE-S). Simultaneous reduction of sulfate and nitrate was significant in BESs supplemented with either sulfate or nitrate during heat-shock pretreatment of the culture. The highest SO42− removal of 88.91 ± 0.8% was found in culture heat pretreated with NO3− and applied with 20 mV potential (BHE-N). The kinetics of heat-pretreated culture showed higher R2 and ultimate potential for H2 on the continuous application of 20 mV potential.




Similar content being viewed by others
Availability of data and materials
On-request data are available.
References
Agostino V, Rosenbaum MA (2018) Sulfate-reducing electroautotrophs and their applications in bioelectrochemical systems. Front in Energ Res 6:55
APHA (2012) Standard methods for the examination of water and wastewater. American Public Health Association, American Water Works Association Water Environment Federation, Washington DC
Aulenta F et al (2012) Linking bacterial metabolism to graphite cathodes: electrochemical insights into the H2-producing capability of Desulfovibrio sp. Chemsuschem 5(6):1080–1085
Bagramyan K, Trchounian A (2003) Structural and functional features of formate hydrogen lyase, an enzyme of mixed-acid fermentation from Escherichia coli. Biochem Mosc 68(11):1159–1170
Bajracharya S et al (2017) Biotransformation of carbon dioxide in bioelectrochemical systems: State of the art and future prospects. J Power Sources 256:256–273
Bhushan KP, Singh R (2020) Bio-electrochemically hydrogen and methane production from co-digestion of wastes. Energy 198:117259
Boboescu IZ et al (2014) Revealing the factors influencing a fermentative biohydrogen production process using industrial wastewater as fermentation substrate. Biotechnol Biofuels 7(1):1–15
Buckel W, Thauer RK (2018) Flavin-based electron bifurcation, ferredoxin, flavodoxin, and anaerobic respiration with protons (Ech) or NAD+ (Rnf) as electron acceptors: a historical review. Front Microbiol 9:401
Cabello P et al (2009) Nitrogen cycle encyclopedia of microbiology. Elsevier, Amsterdam
Cabrol L et al (2017) Microbial ecology of fermentative hydrogen producing bioprocesses: useful insights for driving the ecosystem function. FEMS Microbiol Rev 41(2):158–181
Chang J et al (2016) Simultaneous removals of nitrate and sulfate and the adverse effects of gravel-based biofilters with flower straws added as exogenous carbon source. Ecol Eng 95(189):197
Chen Y, Cheng JJ, Creamer KS (2008) Inhibition of anaerobic digestion process: a review. Biores Technol 99:4044–4064
Costa RB, Bevilaqua D, Lens PN (2020) Pre-treatment and temperature effects on the use of slow release electron donor for biological sulfate reduction. J Environ Manage 275:111216
Croese E et al (2011) Analysis of the microbial community of the biocathode of a hydrogen-producing microbial electrolysis cell. Appl Microbiol Biotechnol 92(5):1083–1093
de Kok S, Meijer J, van Loosdrecht MCM, Kleerebezem R (2013) Impact of dissolved hydrogen partial pressure on mixed culture fermentations. Appl Microbiol Biotechnol 97(6):2617–2625
Dhar V, Singh R (2021) Impact of partially submersed iron scraps in simultaneously sulfate and nitrate removal using sulfate-reducing bacteria. Environ Technol Innov 24:101823
Ding C, Yang KL, He J (2016) Biological and fermentative production of hydrogen. In: Luque R, Lin CSK, Wilson K, Clark J (eds) Handbook of biofuels production. Woodhead Publishing, s.l., pp 303–333
Dykstra CM, Pavlostathis SG (2021) Hydrogen sulfide affects the performance of a methanogenic bioelectrochemical system used for biogas upgrading. Water Res 200:117268
Garcia-de-Lomas J et al (2007) Nitrate stimulation of indigenous nitrate-reducing, sulfide-oxidising bacterial community in wastewater anaerobic biofilms. Water Res 41(14):3121–3131
Gibert O, Pomierny S, Rowe I, Kalin RM (2008) Selection of organic substrates as potential reactive materials for use in a denitrification permeable reactive barrier (PRB). Biores Technol 99(16):7587–7596
Giblin AE et al (2013) The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 26(3):124–131
Gutierrez-Wing MT, Malone RF, Rusch KA (2012) Evaluation of polyhydroxybutyrate as a carbon source for recirculating aquaculture water denitrification. Aquacult Eng 51:36–43
Haroon MF et al (2013) Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500:567–570
He Q et al (2010) Impact of elevated nitrate on sulfate-reducing bacteria: a comparative study of Desulfovibrio vulgaris. ISME J 4(11):1386–1397
Hu Z et al (2013) Nitrogen removal by a nitritation-anammox bioreactor at low temperature. Appl Environ Microbiol 79(8):2807–2812
Hwang J-H et al (2009) Effect of pH and sulfate concentration on hydrogen production using anaerobic mixed microflora. Int J Hydrogen Energy 34(24):9702–9710
Knittel K, Boetius A (2009) Anaerobic oxidation of methane: progress with an unknown process unknown process. Annu Rev Microbiol 63:311–334
Knowles R (1982) Denitrification. Microbiol Rev 46:43–70
Kumar M, Singh R (2020) Sewage water treatment with energy recovery using constructed wetlands integrated with a bioelectrochemical system. Environ Sci Water Res Technol. https://doi.org/10.1039/C9EW00867E
Lévesque V, et al (2011). The use of artificial wetlands to treat greenhouse effluents. In International Symposium on High Technology for Greenhouse Systems: Green Sys2009 893. s.l., s.n., pp. 1185–1192
Lin Y-F, Jing S-R, Lee D-Y, Wang T-W (2002) Nutrient removal from aquaculture wastewater using a constructed wetlands system. Aquaculture 209(1–4):169–184
Liu H, Wang G, Zhu D, Pan G (2009) Enrichment of the hydrogen-producing microbial community from marine intertidal sludge by different pretreatment methods. Int J Hydrog Energ 34(24):9696–9701
Lovely DR (2017) Happy together: microbial communities that hook up to swap electrons. ISME J 11(2):327–336
Luo Y et al (2008) Organic loading rates affect composition of soil-derived bacterial communities during continuous, fermentative biohydrogen production. Int J Hydrog Energ 33(22):6566–6576
Marietou A (2016) Nitrate reduction in sulfate-reducing bacteria. FEMS Microbiol Lett 363(15):155
Martins M, Pereira IAC (2013) Sulfate-reducing bacteria as new microorganisms for biological hydrogen production. Int J Hydrog Energ 38:12294–12301
Moon C, Singh R, Chaganti SR, Lalman JA (2013) Modeling sulfate removal by inhibited mesophilic mixed anaerobic communities using a statistical approach. Water Res 47:2341–2351
Nie W-B et al (2021) Simultaneous nitrate and sulfate dependent anaerobic oxidation of methane linking carbon, nitrogen and sulfur cycles. Water Res 194:116928
Nishimura N, Kitaura S, Mimura A, Takahara Y (1992) Cultivation of thermophilic methanogen KN-15 on H2-CO2 under pressurized conditions. J Ferment Bioeng 73(6):477–480
Ntaikou I, (2021) Microbial production of hydrogen. In: Sustainable fuel technologies handbook. s.l.:Academic Press, p 315-337
Ontiveros-Valencia A et al (2012) Interactions between nitrate-reducing and sulfate-reducing bacteria coexisting in a hydrogen-fed biofilm. Environ Sci Technol 46(20):11289–11298
Osborne JP, Planer J (2011). Nitrate (anaerobic) Pathway Map
Park J, Craggs R, Sukias J (2009) Removal of nitrate and phosphorus from hydroponic wastewater using a hybrid denitrification filter (HDF). Biores Technol 100(13):3175–3179
Patterson BM et al (2002) Use of polymer mats in series for sequential reactive barrier remediation of ammonium-contaminated groundwater: laboratory column evaluation. Environ Sci Technol 36(15):3439–3445
Payne WJ (1973) Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev 37:409–452
Pendyala B et al (2012) Pretreating mixed anaerobic communities from different sources: correlating the hydrogen yield with hydrogenase activity and microbial diversity. Int J Hydrog Energ 37(17):12175–12186
Plugge CM, Zhang W, Scholten JCM, Stams AJM (2011) Metabolic flexibility of sulfate-reducing bacteria. Front Microbiol 2:81
Poudel S et al (2018) Origin and evolution of flavin-based electron bifurcating enzymes. Front Microbiol 9:1762
Ragsdale SW, Pierce E (2008) Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim et Biophys Acta (BBA) Protein Proteom 1784(12):1873–1898
Ricardo AR et al (2012) Kinetics of nitrate and perchlorate removal and biofilm stratification in an ion exchange membrane bioreactor. Water Res 46(14):4556–4568
Rittmann BE, McCarty PL (2001) Environmental biotechnology: principle and application. McGraw-Hill Companies, New York
Sakamoto IK et al (2012) Evaluation of microorganisms with sulfidogenic metabolic potential under anaerobic conditions. Braz Arch Biol Technol 55:779–784
Sander R (2015) Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos Chem Phys 15(8):4399–4981
Singh NK, Singh R (2021) Evaluation of pretreatment potential and hydrogen recovery from lignocellulosic biomass in an anoxic double-staged bioelectrochemical system. Int J Hydrog Energ 46(79):39122–39135
Singh NK, Kumari P, Singh R (2021) Intensified hydrogen yield using hydrogenase rich sulfate-reducing bacteria in bio-electrochemical system. Energy 219:119583
Stams AJ, Oude Elferink SJ, Westermann P (2003) Metabolic interactions between methanogenic consortia and anaerobic respiring bacteria. Adv Biochem Eng Biotechnol 81:31–56
Suri N, Zhang Y, Gieg LM, Ryan MC (2021) Denitrification biokinetics: towards optimization for industrial applications. Front Microbiol 12:795
Thauer RK (2011) Anaerobic oxidation of methane with sulfate: on the reversibility of the reactions that are catalyzed by enzymes also involved in methanogenesis from CO2. Curr Opin Microbiol 14(3):292–299
Thauer RK, Jungermann K, Decker K (1997) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Timmers PH et al (2017) Reverse methanogenesis and respiration in Methanotrophic archaea. Archaea 22:1654237
Varma SK, Singh R (2022) RB-based bioelectrochemical system: a potential multipollutant combatant for enhanced landfill waste stabilization. Waste Manage 154:1–14
Wang K et al (2017) Impact of applied current on sulfate-rich wastewater treatment and microbial biodiversity in the cathode chamber of microbial electrolysis cell (MEC) reactor. Chem Eng J 307:150–158
Warneke S et al (2011) Rates, controls and potential adverse effects of nitrate removal in a denitrification bed. Ecol Eng 37(3):511–522
Wen Y et al (2010) Effects of plant biomass on nitrate removal and transformation of carbon sources in subsurface-flow constructed wetlands. Biores Technol 101:7286–7292
Whitmire SL, Hamilton SK (2005) Rapid removal of nitrate and sulfate in freshwater wetland sediments. J Environ Qual 34(6):2062–2071
Wu S et al (2013) Sulphur transformations in constructed wetlands for wastewater treatment: a review. Ecol Eng 52:278–289
Xiang Y et al (2017) Acetate production and electron utilization facilitated by sulfate-reducing bacteria in a microbial electrosynthesis system. Biores Technol 241:821–829
Xu XJ et al (2014) Kinetics of nitrate and sulfate removal using a mixed microbial culture with or without limited-oxygen fed. Appl Microbiol Biotechnol 98(13):6115–6124
Xu H et al (2018) Simultaneous autotrophic removal of sulphate and nitrate at different voltages in a bioelectrochemical reactor (BER): evaluation of degradation efficiency and characterization of microbial communities. Biores Technol 265:340–348
Yavuz B, Türker M, Engin G, l. Ö. (2007) Autotrophic removal of sulphide from industrial wastewaters using oxygen and nitrate as electron acceptors. Environ Eng Sci 24(4):457–470
Zeng Y, Priest C, Wang G, Wu G (2020) Restoring the nitrogen cycle by electrochemical reduction of nitrate: progress and prospects. Small Methods 4(12):2000672
Zhang L et al (2008) Chemical and biological technologies for hydrogen sulfide emission control in sewer systems: a review. Water Res 42:1–12
Zhao H-P et al (2013) Using a two-stage hydrogen-based membrane biofilm reactor (MBfR) to achieve complete perchlorate reduction in the presence of nitrate and sulfate. Environ Sci Technol 47(3):1565–1572
Zwietering MH, Jongenburger I, Rombouts FM, Riet KV (1990) Modeling of the bacterial growth curve. Appl Environ Microbiol 56:1875–1881
Acknowledgements
One of the authors Dhar V. is grateful to the University Grants Commission (UGC), New Delhi for providing a Non-NET Fellowship. We also thank Mr. Kailash Bahuguna (Chief Operating Officer, Zydus Infrastructure Pvt. Ltd.) for providing the anaerobic sludge and other technical help required for the research study.
Funding
There was no funding provided for the work.
Author information
Authors and Affiliations
Contributions
VD: Investigation, methodology, data curation, formal analysis and data interpretation, writing—original draft. RS: Methodology, Writing—review and editing, validation, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethics approval
No ethical approval is required for this study.
Consent to participate
Not applicable.
Consent of publication
Not applicable.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dhar, V., Singh, R. Biohydrogen production potential with sulfate and nitrate removal by heat-pretreated enriched sulfate-reducing microorganisms-based bioelectrochemical system. Arch Microbiol 205, 7 (2023). https://doi.org/10.1007/s00203-022-03352-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03352-4