Abstract
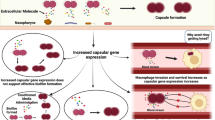
Neisseria meningitidis is a commensal of human nasopharynx which under certain unidentified conditions could lead to fulminant meningitis or sepsis. Availability of nutrients is essential for bacterial growth and virulence. The metabolic adaptations allow N. meningitidis to utilize host resources, colonize and cause virulence functions which are a crucial for the invasive infection. During colonization meningococci encounters a range of microenvironments involving fluctuations in the availability of carbon and nitrogen source. Therefore, the characterization of virulence factors of N. meningitidis under different microenvironmental conditions is a prime requisite to understand pathogenesis; however, the role of nutrients is not well understood. Here, we explore the expression of virulence phenotype leading to symptomatic behaviour as affected by available carbon and nitrogen sources. We evaluate the effect of carbon or nitrogen source on growth, adhesion to epithelial cells, macrophage infectivity, capsule formation and virulence gene expression of N. meningitidis. It was found that lactate, pyruvate, and acetate facilitate survival of N. meningitidis in macrophages. While in epithelial cells, the survival of N. meningitidis is negatively affected by the presence of lactate and pyruvate.




Similar content being viewed by others
Data availability
Data could be available from the corresponding author on reasonable request.
References
Bartley SN, Tzeng YL, Heel K et al (2013) Attachment and invasion of Neisseria meningitidis to host cells is related to surface hydrophobicity, bacterial cell size and capsule. PLoS ONE 8:e55798
Blanchette KA, Shenoy AT, Milner J et al (2016) Neuraminidase A-exposed galactose promotes Streptococcus pneumoniae biofilm formation during colonization. Infect Immun 84:2922–2932
Brimacombe CA, Beatty JT (2013) Surface polysaccharide extraction and quantification. Bio-Protoc 3:e934–e934
Brown SA, Palmer KL, Whiteley M (2008) Revisiting the host as a growth medium. Nat Rev Microbiol 6:657–666
Caugant DA, Maiden MC (2009) Meningococcal carriage and disease-population biology and evolution. Vaccine 27:B64–B70
Chien AC, Zareh SK, Wang YM et al (2012) Changes in the oligomerization potential of the division inhibitor UgtP co-ordinate Bacillus subtilis cell size with nutrient availability. Mol Microbiol 86:594–610
Derkaoui M, Antunes A, Abdallah JN et al (2016) Transport and catabolism of carbohydrates by Neisseria meningitidis. J Mol Microbiol Biotechnol 26:320–332
Exley RM, Goodwin L, Mowe E, Shaw J, Smith H, Read RC, Tang CM (2005) Neisseria meningitidis lactate permease is required for nasopharyngeal colonization. Infect Immun 73:5762–5766
Frantz ID Jr (1942) Growth requirements of the meningococcus. J Bacterial 43:757–761
Hill DJ, Griffiths NJ, Borodina E et al (2010) Cellular and molecular biology of Neisseria meningitidis colonization and invasive disease. Clin Sci 118:547–564
Kalsi KK, Baker EH, Medina RA et al (2008) Apical and basolateral localisation of GLUT2 transporters in human lung epithelial cells. Pflüg Arch-Eur J Phy 456:991–1003
Kwaik YA, Bumann D (2013) Microbial quest for food in vivo: ‘Nutritional virulence’ as an emerging paradigm. Cell Microbiol 15:882–890
Laver JR, Hughes SE, Read RC (2015) Neisserial molecular adaptations to the nasopharyngeal niche. Adv Microb Physiol 66:323–355
Leighton MP, Kelly DJ, Williamson MP et al (2001) NMR and enzyme study of the carbon metabolism of Neisseria meningitidis. Microbiology 147:1473–1482
Merz AJ, So M (2000) Interactions of pathogenic neisseriae with epithelial cell membranes. Ann Rev Cell Dev Bio 16:423–457
Nassif X, Beretti JL, Lowy J, Stenberg P et al (1994) Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells. Proc Natl Acad Sci 91:3769–3773
Pagliarulo C, Salvatore P, De Vitis LR et al (2004) Regulation and differential expression of gdhA encoding NADP-specific glutamate dehydrogenase in Neisseria meningitidis clinical isolates. Mol Microbiol 51:1757–1772
Read RC, Zimmerli S, Broaddus C et al (1996) The (alpha2–> 8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect Immun 64:3210–3217
Schoen C, Kischkies L, Elias J, Ampattu BJ (2014) Metabolism and virulence in Neisseria meningitidis. Front Cell Infect Microbiol 4:114
Sigurlásdóttir S, Engman J, Eriksson OS et al (2017) Host cell-derived lactate functions as an effector molecule in Neisseria meningitidis microcolony dispersal. PLoS Pathog 13(4):e1006251
Sigurlásdóttir S, Saroj SD, Eriksson O et al (2018) Quantification of Neisseria meningitidis adherence to human epithelial cells by colony counting. Bio Protoc 8:1–10
Smith H, Parsons NJ, Cole JA (1995) Sialylation of neisserial lipopolysaccharide: a major influence on pathogenicity. Microb Pathog 19:365–377
Smith H, Yates EA, Cole JA, Parsons NJ (2001) Lactate stimulation of gonococcal metabolism in media containing glucose: mechanism, impact on pathogenicity, and wider implications for other pathogens. Infect Immun 69:6565–6572
Smith H, Tang CM, Exley RM (2007) Effect of host lactate on gonococci and meningococci: new concepts on the role of metabolites in pathogenicity. Infect Immun 75:4190–4198
Snyder LA, Saunders NJ (2006) The majority of genes in the pathogenic Neisseria species are present in non-pathogenic Neisseria lactamica, including those designated as’ virulence genes’. BMC Genomics 7:1–11
Soriani M (2017) Unraveling Neisseria meningitidis pathogenesis: from functional genomics to experimental models. F1000 6:1228
Stephens DS (2009) Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis. Vaccine 27:B71–B77
Stephens DS, Spellman PA, Swartley JS (1993) Effect of the (α2→ 8)-linked polysialic acid capsule on adherence of Neisseria meningitidis to human mucosal cells. J Infect Dis 167:475–478
Svennerholm L (1957) Quantitative estimation of sialic acids: II A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta 24:604–611
Tzeng YL, Stephens DS (2000) Epidemiology and pathogenesis of Neisseria meningitidis. Microbes Infect 2:687–700
Van Deuren M, Brandtzaeg P, van der Meer JW (2000) Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev 13:144–166
Virji M (2009) Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nat Rev Microbiol 7:274–286
Virji M, Alexandrescu C, Ferguson DJ, Saunders JR, Moxon ER (1992) Variations in the expression of pili: the effect on adherence of Neisseria meningitidis to human epithelial and endothelial cells. Mol Microbiol 6:1271–1279
Virji M, Makepeace K, Ferguson DJ, Achtman M, Moxon ER (1993) Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol Microbiol 10:499–510
Virji M, Makepeace K, Peak IR, Ferguson DJ, Jennings MP, Moxon ER (1995) Opc-and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol Microbiol 18:741–754
Yi K, Rasmussen AW, Gudlavalleti SK, Stephens DS, Stojiljkovic I (2004) Biofilm formation by Neisseria meningitidis. Infect Immun 72:6132–6138
Acknowledgements
PK is supported by the junior research fellowship program of the Symbiosis International (Deemed University).
Funding
The work was supported by the Ramalingaswami fellowship program of Department of Biotechnology, India under Grant BT/RLF/Re-entry/41/2015.
Author information
Authors and Affiliations
Contributions
PK and RJ prepared the first draft; PK and RJ performed the assays and analysed the data. SD conceptualized the idea, examined and analysed data. PK and SD finalized the draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethics approval
No animals were used in the experiments. Therefore, ethics approval was not required.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kanojiya, P., Joshi, R. & Saroj, S.D. The source of carbon and nitrogen differentially affects the survival of Neisseria meningitidis in macrophages and epithelial cells. Arch Microbiol 204, 404 (2022). https://doi.org/10.1007/s00203-022-03037-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03037-y