Abstract
This investigation tested the hypothesis that the native cyanobacteria can acclimatize and grow under the combination of environmental factors and/or how does their process change with the age of culture? Here, we tried to combine multiple factors to simulated what happens in natural ecosystems. We analyzed the physiological response of terrestrial cyanobacterium, Cylindrospermum sp. FS 64 under combination effect of different salinity (17, 80, and 160 mM) and alkaline pHs (9 and 11) at extremely limited carbon dioxide concentration (no aeration) up to 96 h. Our evidence showed that growth, biomass, photosystem II, and phycobilisome activity significantly increased under 80 mM salinity and pH 11. In addition, this combined condition led to a significant increase in maximum light-saturated photosynthesis activity and photosynthetic efficiency. While phycobilisomes and photosystem activity decreased by increasing salinity (160 mM) which caused decreased growth rates after 96 h. The single-cell study (CLMS microscopy) which illustrated the physiological state of the individual and active-cell confirmed the efficiency and effectiveness of both photosystems and phycobilisome under the combined effect of 80 mM salinity and pH 11.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria are a self-sufficient system that is widely distributed across terrestrial and aquatic environments; and terrestrial cyanobacteria plays a fundamental role in the biological cycle of agriculture (Mareš et al. 2014; Shokravi and Bahavar 2021a). They produce various bio-available elements such as Nitrogen and phosphorus, which are the essential nutrients for plant cultivation (Chittora et al. 2020). Moreover, they generally exhibit a high level of adaptive abilities and tolerance to a large number of environmental factor (Singh 2018). In nature, cyanobacteria are exposed to a constantly changing environment including irradiance, temperature, pH, nutrient availability, salinity, dissolved inorganic carbon fluctuations (Chris et al. 2006; Bouazzara et al. 2020). These changes continuously expose the cyanobacteria cells to multiple stressors of varying magnitude and duration (Borowitzka 2018). In practice, the survival and growth of cyanobacteria depend on their ability to acclimate varying environmental conditions.
Among all cultural parameters, pH is one of the most important factors determining cyanobacteria growth and physiology (Pawlik-Skowrońska et al. 1997; Hinners et al. 2015). Most cyanobacteria have the ability to grow over a wide range of alkaline pH in laboratory (Kaushik 1994). Nearly nothing is known about the mechanisms of cellular survival, cell stability, or growth of cyanobacteria under extreme alkaline conditions (Jangir et al. 2021). Elevated pH (alkalinity) directly influences growth rate and cell yield (de Souza Santos et al. 2011; Shokravi and Bahavar 2021a, b), enzyme activity (Li et al. 2013), biosorption (El-Din 2017), resistance to oxidative stress (Summerfield et al. 2013), and protection (Pathak et al. 2018). It also strongly affects the cyanobacterial abundance (Krausfeldt et al. 2019; Nguyen and Rittmann 2015). While in some cyanobacteria, the growth decreased with the increasing alkalinity (Shokravi and Bahavar 2021a). Therefore, evaluation the effects of different pHs on cyanobacteria is important. In paddy fields, the pH of floodwater varies during the day. Likewise, DIC concentration in the floodwater varies daily and seasonally depending on photosynthetic and respiratory rate (Pedersen et al. 2013). The chemical equilibrium between photosynthesis and respiration implies a balance between inorganic carbon and net ecosystem production (Khan et al. 2020).
Salinity is another environmental factor that could potentially determine the cyanobacteria community in natural ecosystems. Salinity as an essential factor induces diverse alterations in the growth and photosynthesis (Bemal and Anil 2018), biochemical like carbohydrate content (Singh et al. 2015), and physiological characteristics of cyanobacteria (Miriam et al. 2017; Lee et al. 2021). Time (age of the culture) is another essential factor in the resistance and growth in different conditions (Alcorta et al. 2019) which less has been considered (Bouazzara et al. 2020; Jangir et al. 2021). Exposure to initial hours of new condition may create a significant effect on physiological activities during the next hours (Abbasi et al. 2019; Shokravi and Bahavar 2021a). However, there is increasing evidence that the combined environmental factors can be modulated by other factors and led to regulation, acclimation, and adaptation (Müller et al. 1993; Shokravi and Bahavar 2021a). Therefore, studying environmental fluctuations in the short-time regime on cyanobacteria is essential to serve the sustainable development economy in the future.
In the present study, we have selected the filamentous cyanobacterium Cylindrospermum sp. for the abundance, fixed Nitrogen, and environmental stability. Cylindrospermum sp. FS 64 was isolated from paddy fields of the North of Iran which description is following: aggregations bright blue-green, green–brown, sticky colonies, not regular, expanded; mucilaginous, attached to margin; filaments straight, curved entangled; trichrome 9µ broad, constricted at cross wall; cells variable in size, cylindrical or nearly quadrate; heterocyst ovate or ellipsoid, terminal at one side; spore oval, ellipsoidal, adjoining the heterocyst, granular, 10.4µ long.
So far, most studies on this genus have been focused on molecular biology (Srivastava et al. 2009; Katoch et al. 2016), physiological characteristics (Briand et al. 2004), proline accumulation (Chris et al. 2006), heavy metal stress (Singh et al. 1989; Chris 2012), Nitrogen forms effect (Kenesi et al. 2009) and chemical analysis (Mareš et al. 2014). The aim of this research is to characterize the acclimation behaviors of Cylindrospermum sp. FS 64 under different pH, salinity conditions under extremely limited carbon dioxide concentration for 96 h.
Materials and methods
Culture maintenance and growth conditions
Cylindrospermum sp. FS 64 was isolated from paddy fields of the North of Iran (Siahbalaei et al. 2011) and collected again by the authors in 2018. The soil samples was serially diluted in sterilized liquid Nitrogen-free medium (BG-110) (Stanier et al. 1971). Isolation was done by streaking and spreading technique on solid BG-110 medium. Purification was done by alternative sub-culturing between liquid and solid BG-110 medium (Shokravi and Bahavar 2021b). The sample was identified and described using multidisciplinary approaches (Molecular 16S rRNA, and morphology using light, fluorescence, and phase-contrast microscopy). Strain after identification as Cylindrospermum sp. FS 64 was coded and preserved in the algae museum of the institute of applied sciences of Shahid Beheshti University, Tehran-Iran. The axenic culture were maintained in a liquid BG-110 at temperature 30 ± 2 °C under a constant irradiance of 60 μmol quanta m−2 s−1 (Poza-Carrión et al. 2001). The pH was adjusted in 7.8 by NaOH.
Growth conditions and analysis
Growth of Cylindrospermum sp. FS 64—in an exponential growth phase—was carried out at various salinity concentrations 17 (culture media without NaCl), 80 and 160 mM at alkaline pHs (9 and 11). Culture media were buffered with 10 mM BTP (Bis–Tris Propane) for pH 9 and 11 adjusted to the desired pH with KOH (Shokravi and Soltani 2011). We studied cultures without CO2 or O2 bubbling and stirring (standing condition, extremely DIC limitation) (Poza-Carrión et al. 2001; Shokravi and Bahavar 2021a). The determination of the growth was performed using time-course measurements by the correlation between optical density (OD 750 nm), in vivo fluorescence, and counting cells according to Briand et al. (2004) using the CLMS at different salinity and alkaline pHs up to 96 h. The OD was measured using Synergy HTX (Multi-Mode Microplate Reader, USA). Growth rates (µ) were calculated according to Li et al. (2014) and Khazi et al. (2018). The absorbance of Chlorophyll content was determined spectrophotometrically at 665 nm according to Marker (1972).
Physiological characterization
To survey the photosynthetic activity and respiratory electron transport chains under different salinity and alkaline conditions, oxygen exchange was studied. Steady-state oxygen evolution was measured with a Clark-type electrode PSII activity in whole cells. Cells cultured at temperature 30 ± 2 °C and constant illumination 60 μmol quanta m−2 s−1 (Inoue-Kashino et al. 2005). The amount of liberated oxygen was normalized by the amount of chlorophyll according to Poza-Carrion (Poza-Carrión et al. 2001). The initial physiological status of Cylindrospermum sp. FS 64 was performed by measuring the maximum photosynthetic rate (Pmax) and photosynthetic efficiency (α) and light saturation (Ik) values after growth analysis. Photosynthesis–irradiance (P–I) curves were calculated by measuring oxygen evaluation rates during successive 1-min illumination periods with a stepwise increase from 0 to 2500 μmol quanta m−2 s−1. The photosynthetic pigments were estimated in terms of chlorophyll a, phycocyanin, from 380 to 760 nm using Synergy HTX (Multi-Mode Microplate Reader, USA) and they normalized to optical density according to Tang and Vincent (1999). The operation of photosystems and phycobilisomes characteristics were analyzed spectrofluorimetrically according to Inou-Kashino et al. (2005), Vermaas et al. (2008) and Zorz et al. (2015). Room temperature fluorescence emission spectra of the cells were recorded following Tiwari and Mohanty (1996) and Fraser et al. (2013). The excitation spectra were recorded at λex: 440 to excite chlorophyll a and 550 nm for phycocyanin. The single-cell study (the fluorescence intensity of single cell) which illustrated the physiological state of the individual, live-cell and spectral unmixing (Grigoryeva and Chistyakova 2019) was measured using λ scan of confocal laser microscope system (Leica TCS-SP5 CLSM—Leica Microsystems Heidelberg GmbH, Mannheim, Germany). Photosynthetic pigment excitation was carried out with an argon laser at 405 nm. The fluorescence emission spectrum was collected by detecting wavelengths between 415 and 760 (Ramírez et al. 2011; Sugiura and Itoh 2012; Shokravi and Bahavar 2021a, b). Analysis of the lambda scan data was carried out using the Leica Confocal Software.
Statistical analysis
Analysis of variance (ANOVA) with the SPSS-24 software was used to evaluate the results. The ANOVA showed a significant difference between treatments with p < 0.05. All the experiments were carried out in six independent biological replicates. For relationships of photosynthesis activity, growth and age of cultures, we fitted a model to the data using interpolation in MATLAB software.
Results
Growth
This study evaluated—in vivo experiments—the ability of acclimation and growth of Cylindrospermum sp. FS 64 under multiple environmental factors at a short period of time under extreme DIC limitation. In general, the results supported the hypothesis that the combined environmental factors can be led to growth and acclimation in different environmental conditions at short time. Comparison of the growth curve of Cylindrospermum sp. FS 64 showed that extreme alkaline condition (pH 11) was more favorable to growth and had a significant effect (p < 0.05) on biomass production compared to pH 9 under extreme DIC limitation (Fig. 1). A study of the length of the incubation period revealed that 80 mM salinity caused significantly growth increased at pH 11 after 48 h and, likewise no significant effect was observed between 17 and 80 mM salinity at pH 11 after 96 h. Regardless of salinity, pH 11 was the optimum pH in this strain up to 168 h (Result not shown). The presence of 160 mM salinity at both alkaline pHs caused significant inhibition of growth and biomass production until 96 h. The metabolic activities and synthesizing enzymes in the growth stage led to the shorter lag phase under alkaline pHs. Therefore, cells acclimatized in both combined conditions 17 mM—pH 9, and 80 mM—pH 11 after 24 h.
Photosynthesis (photosynthetic oxygen evolution and P–I curve)
Regardless of salinity and age of the cultures, the maximum rates of oxygen evolution of Cylindrospermum sp. FS 64 gradually increased at pH 11 (~ 100–250 µmol O2 mg Chl a−1 × h−1) against pH 9 (~ 120–160 µmol O2 mg Chl a−1 × h−1) (Fig. 2). The combined effect of salinity, alkalinity and age of culture revealed that the maximum rates of oxygen evolution significantly increased at pH 11 and 80 mM salinity after 48 h (Fig. 2b). In contrast, the significant decrease was observed under high salinity (160 mM) after 72 h at pH 9. Our purpose of the fitting model was to examine the relationships and quantitative description between photosynthesis, growth, and age of culture (Fig. 3). We observed a significant increase in growth and biomass production and photosynthesis activity under the combined environmental factors. A positive and significant correlation was found under the combined 80 mM salinity and pH 11 after 96 h.
The combined effects of environmental factors on photosynthetic parameters (P–I) are summarized in Table 1. The maximum value of the photosynthesis activity (Pmax)—indicating carboxylation or a step closely associated with carboxylation—was approximately 90% higher at combined 80 mM salinity and pH 11 compared to pH 9. Ik indicating the irradiance at which control of photosynthesis passes from light absorption and photochemical energy conversion to reductant utilization (Sakshaug et al. 1997). Ik was lower at the combination of low salinity (17 and 80 mM) and pH 11 compared to pH 9, indicating that the rate of water oxidation in PSII was reduced. α is often used for comparing the cyanobacteria shade endurance (shade tendency) under shading conditions. Noticeably, in the presence of 80 mM salinity, the shade-adapted capacity of cyanobacterium significantly increased at both alkaline pHs.
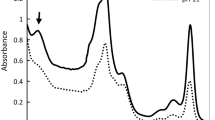
Absorption spectra
In vivo absorbance spectroscopy as a common method to obtain an overview of the content and distribution of the pigments of cells (Fig. 4) showed that chlorophyll Soret bands (~ 445 nm), chlorophyll a of PSII (~ 680 nm), and phycocyanin (~ 620–630 nm) was the principal active compound in all treatments. A shoulder at ~ 495 nm related to carotenoids has appeared. In addition, we observed an increase in absorption of ~ 250–300 nm which is most probably related to one of the carotenoids bands (generally, organic molecules) under the combination of pH 11 and 80 mM salinity. Our results revealed that the dynamism and stability of PSII and phycobilisomes significantly increase in the combined effect of 80 mM salinity and pH 11 compared to pH 9 after 72 h. While no significant differences were observed between 17 and 80 mM salinity at pH 9. In presence of 160 mM salinity, the structure and stability of phycocyanin and PSII were demolished at pH 11 after 72 h, although they are maintained their structure up under pH 9. In addition, the absence of the shift and stability of the Chl a of PSII was noticeable in all treatments.
PBS and PSII stability under different salinity and alkaline pHs
We investigated the distribution of energy between phycobiliproteins and PSII spectrofluorimetrically at excitation 440 nm (chlorophyll- associated with PSII), and 550 (phycocyanin). We observed addition of 80 mM salinity was accompanied by increasing PSII (Fig. 5) and phycocyanin activity (Fig. 6) at pH 11 compared to pH 9. Although, increasing salinity (160 mM) drastically decreased the efficiency and effectiveness of PSII and PC activity at both alkaline pHs after 24 h (Figs. 5, 6). This study confirmed the high growth, biomass production, and content of the PSII and PBS (absorption spectra) under the combined effect of 80 mM salinity at pH 11.
Spectroscopic study of a single cell by CLSM
Investigation of single cell illustrated the physiological state of the individual, live-cell and maximum fluorescent of pigment protein which enabled us to monitor the dynamic processes of the chosen cells as spectral unmixing and all steps of the energy transfer chain (Grigoryeva and Chistyakova 2019; Shokravi and Bahavar 2021b) (Fig. 7). We observed the high fluorescence of Chl a (PSII) at ~ 680 nm and PSI at ~ 715 nm at vegetative cells compared to heterocyst—because of low amounts of PSII and PBS at heterocyst (heterocyte result not shown). In addition, a clear shoulder was observed around 663 nm related to the APC (Allophycocyanin) of phycobilin production, which was highest at 80 mM salinity at pH 11. Increasing salinity (160 mM) led to declined APC content at both alkaline pHs. This reduction which was saline dependent caused the demolished structure of PBS at pH 9 compared to pH 11. Chl a (PSII-680 nm) was the most stable pigment under all conditions, and it was higher under the combined 80 mM salinity and pH 11. No significant difference of PSII and also PSI activity was observed between 17 and 80 mM salinity after 72 h at pH 9 against pH 11. While the highest PSI activity belongs to 80 mM salinity at pH 11. Overall, our results of the single-cell study confirmed the highest growth and stability of PS and PBS depends on combined 80 mM salinity and pH 11 (Figs. 1, 5, 6).
Discussion
Overall, our results provide important insight that combined multiple factors such as salinity, alkalinity, and the age of the cultures in laboratory conditions plays a key role in acclimatizing the growth, and photosynthesis of Cylindrospermum sp. FS 64. Absorption spectra and chlorophyll concentration (OD 750) methods as an overview of growth and cells activity on the culture (Schulze et al. 2011) indicated that salinity and alkalinity (combine together—pH 11 and 80 mM salinity) cannot be considered as stress to limit the growth (Borowitzka 2018) of Cylindrospermum sp. FS 64. Low salinity (17 and 80 mM) in heterocystous cyanobacteria (Srivastava et al. 2009) can be desired as nutrition and stimulant leads to a significant increase in growth, biomass production (Miriam et al. 2017), and photosynthesis operation (Singh et al. 2015). The elevated salinity (160 mM) at both alkaline pHs 9 and 11 led to inhibition of Chl biosynthesis (Chris et al. 2006) which resulted in a decrease in chlorophyll pigment. Besides, these findings are in line with our other study on Nostoc sp. UAB 206 that isolated from the Spanish paddy field (not published data). Valiente and Leganes (1990), Poza-Carrión et al. (2001), Soltani et al. (2006), Amirlatifi et al. (2018), Abbasi et al. (2019) and Shokravi and Bahavar (2021a) have indicated that the optimum pH for growth, photosynthesis and nitrogen fixation of terrestrial cyanobacteria under combined environmental factors is about 8 or 9. The response of strain to pH 11 and 80 mM salinity may depend on the high genetic plasticity (Boussiba et al. 2000) or and is an inherent characteristic (Tang and Vincent 1999) which resulted in without any requirement for an acclimation process (Vonshak and Torzillo 2007). Furthermore, we observed a strong correlation-fitting model—under the combined 80 mM salinity and pH 11 up to 96 h which confirmed the highest activity of cells under this condition. Pigment analysis of cell cultures (absorption spectra) also confirmed the results of the activity of the cells under this condition.
No accurate understanding of the mechanisms involved in pH homeostasis in cyanobacteria. Giraldez-Ruiz et al. (1997) proposed that Na, K, and Ca could be considered as part of the pH homeostatic system. Touloupakis et al. (2016) reported that many mechanisms have been suggested for pH homeostasis and the regulation of CO2/HCO3 concentration. Most carbon sources are in bicarbonate ions under severe carbon dioxide deficiency in alkaliphilic cyanobacteria (Boyd 2015). The carbon dioxide concentration mechanism (CCM) is the key process that enables them to acclimate to alkaline conditions (Klanchui et al. 2017). The operation of CCM involves a high amount of energy and naturally requires a high operation of photosynthesis—along with other needs—and high efficiency of PSI, PSII, and PBS (Mangan and Brenner 2014). Regarding the highest growth and photosynthesis at pH 11, Cylindrospermum sp. FS 64 have a powerful carbon dioxide concentration mechanism and flexibility to induce.
The main reasons of growth measuring is understanding the balance between photosynthesis and respiration (Nygård and Dring 2008). The oxygen liberation (a marker of PSII activity) analysis confirmed the growth results. Regardless of salinity, the maximum photosynthesis (Pmax) of Cylindrospermum sp. FS 64 was higher at pH 9 against pH 11. Addition salinity (80 mM) caused an increase in Pmax (Ye and Gao 2004; Dhiab et al. 2007) at pH 11 which can be attributed to the high efficiency of water oxidation in PSII. In contrast, 80 mM salinity led to decreasing in saturating irradiance and increased shade-adapted capacity of strain at both alkaline pHs, which influenced an increase in the relative content of PSII activity and the antenna size of PS II (Inoue-Kashino et al. 2005). Briand et al. (2004) reported that Ik is the most reliable parameter for assessing and comparing the variable-light requirement. The high Ik value of Cylindrospermum sp. FS 64 can be ascribed to the different media, pH, and salinity used, implying an increase in energy for growth.
To better understanding PBS and PS activity and stability, we have used the fluorescence assay. The strain showed nearly 90% of PBS and PSII stability under the combination of 80 mM salinity and pH 11 after 96 h compared to pH 9. While increasing salinity (160 mM) led to demolished of the PBS and PSII structure at both alkaline pHs after 24 h. Galetović et al. (2020) reported most research focuses on PBS behavior and stability in different temperature and pH 5–7 (Antelo et al. 2008), pH 2.0, 6.5 and 8.0 (Couteau et al. 2004) and pH range of 4–9 (Leu et al. 2013). Chris et al. (2006) found a decrease in growth, chlorophyll content, carotenoid, phycocyanin, and PS II activity of Cylindrospermum sp. due to individual salinity as well as in combination with UV-B treatments. In addition, Srivastava et al. (2009) investigated that 150 mM salinity and pH 7.5 caused a decline in PSI, PS II, and whole chain activities in Anabaena doliolum after 24 h. The difference between these findings may be due to the use of various pH ranges which influences the growth, metabolism, regulation, and distribution of cyanobacteria (Jin and Kirk 2018).
During cultivation, chlorophyll content decreases due to environmental factors or dying of the part of the population (age of culture). (Schulze et al. 2011; Shokravi and Bahavar 2021a, b). Therefore, single-cell study is a new method that provide information on the dynamic behavior of each cell (Sugiura and Itoh 2012; Grigoryeva and Chistyakova 2019). By confocal laser microscopy, Ying et al. (2002), Wolf and Schüßler (2005) and Sugiura and Itoh (2012) demonstrated different fluorescence spectra of vegetative and heterocyte cells-unmixing spectra. The results of single-cell spectra support that combination 80 mM salinity and pH 11 up to 96 h led to a noticeable increase and stability in all parts of the phycobilisome and PSII activity. This stability of PSII may depend on the physical change in enzymes and binding sites in PSII and potent PSII efficiency. Reduction of PBS and PSII content indicating the degradation of their structure by increasing salinity at both alkaline pHs. We observed a decline and the shifted peak of PSI (719 nm) that is affected by the lower PC content at pH 9 compared to pH 11. Therefore, cells might accept less excitation energy when PC is reduced (Schmitt et al. 2020).
Conclusion
In conclusion, the different methods confirmed that combining environmental factors (different alkaline pH, salinity, and time under extreme DIC limitation) can affect cyanobacterial behaviors individually or in combination and led to regulation and acclimation in short time. We observed Cylinrospermum sp. FS 64 acclimatized through different strategies and has developed a mechanism for the highest growth, photosystems operation, phycobilisomes activity and light-saturated photosynthetic under 80 mM salinity and pH 11. Conversely, elevated salinity was time dependent at both alkaline conditions. Several lines of evidence supported this issue. From an applied point of view, this cyanobacterium can be used in alkaline–saline paddy fields and agricultural lands as a biofertilizer, soil conditioners, and other biotechnological purposes.
Abbreviations
- APC:
-
Allophycocyanin
- Chl a :
-
Chlorophyll a
- CCM:
-
Carbon dioxide concentrating mechanism
- DIC:
-
Dissolved inorganic carbon
- PBS:
-
Phycobilisome
- PSI, PSII:
-
Photosystems I and II
- CLSM:
-
Confocal laser scanning microscopy
References
Abbasi B, Shokravi S, Golsefidi MA, Sateiee A, Kiaei E (2019) Effects of alkalinity, extremely low carbon dioxide concentration and irradiance on spectral properties, phycobilisome, photosynthesis, photosystems and functional groups of the native cyanobacterium Calothrix sp. ISC 65. Int J Algae 29(1):40–58. https://doi.org/10.1540/alg29.01.040
Alcorta J, Vergara-Barros P, Antonaru LA, Alcamán-Arias ME, Nürnberg DJ, Díez B (2019) Fischerella thermalis: a model organism to study thermophilic diazotrophy, photosynthesis and multicellularity in cyanobacteria. Extremophiles 23(6):635–647. https://doi.org/10.1007/s00792-019-01125-4
Amirlatifi HS, Shokravi S, Sateei A, Golsefidi MA, Mahmoudjanlo M (2018) Samples of cyanobacterium calothrix sp. ISC 65 collected from oil polluted regions respond to combined effects of salinity, extremely low-carbon dioxide concentration and irradiance. Int J Algae. https://doi.org/10.1615/InterJAlgae.v20.i2.80
Antelo FS, Costa JAV, Kalil SJ (2008) Thermal degradation kinetics of the phycocyanin from Spirulina platensis. Biochem Eng J 41(1):43–47. https://doi.org/10.1016/J.BEJ.2008.03.012
Bemal S, Anil AC (2018) Effects of salinity on cellular growth and exopolysaccharide production of freshwater Synechococcus strain CCAP1405. J Plankton Res. https://doi.org/10.1093/plankt/fbx064
Borowitzka MA (2018) The ‘stress’ concept in microalgal biology—homeostasis, acclimation and adaptation. J Appl Phycol 30(5):2815–2825. https://doi.org/10.1007/S10811-018-1399-0
Bouazzara H, Benaceur F, Chaibi R, Boussebci I, Bruno L (2020) Combined effect of temperature, pH and salinity variation on the growth rate of Gloeocapsa sp. in batch culture method using Aiba and Ogawa medium. Eurasian J Biosci 14(2):7101–7109
Boussiba S, Wu X, Zarka A (2000) Alkaliphilic cyanobacteria. In: Seckbach J (ed) Journey to diverse microbial worlds. Cellular Origin and Life in Extreme Habitats, vol 2. Springer, Dordrecht. https://doi.org/10.1007/978-94-011-4269-4_15
Boyd CE (2015) pH, carbon dioxide, and alkalinity. In: Water quality. Springer, Cham, pp 153–178. https://doi.org/10.1007/978-3-319-17446-4_8
Briand JF, Leboulanger C, Humbert JF, Bernard C, Dufour P (2004) Cylindrospermopsis raciborskii (Cyanobacteria) invasion at mid-latitudes: selection, wide physiological tolerance, or global warming? J Phycol 40(2):231–238. https://doi.org/10.1111/j.1529-8817.2004.03118.x
Chittora D, Meena M, Barupal T, Swapnil P (2020) Cyanobacteria as a source of biofertilizers for sustainable agriculture. Biochem Biophys Rep 22:100737. https://doi.org/10.1016/j.bbrep.2020.100737
Chris A (2012) Effect of nickel stress on growth and antioxidants in cyanobacterium Cylindrospermum sp. Asian J Bio Sci 7(1):13–17
Chris A, Zeeshan M, Abraham G, Prasad SM (2006) Proline accumulation in Cylindrospermum sp. Environ Exp Bot 57(1–2):154–159. https://doi.org/10.1016/j.envexpbot.2005.05.008
Couteau C, Baudry S, Roussakis C, Coiffard LJM (2004) Study of thermodegradation of phycocyanin from Spirulina platensis. Sci Aliment 24(5):415–422
de Souza Santos KR, Jacinavicius FR, Sant’Anna CL (2011) Effects of the pH on growth and morphology of Anabaenopsis elenkinii Miller (Cyanobacteria) isolated from the alkaline shallow lake of the Brazilian Pantanal. Fottea 11(1):119–126. https://doi.org/10.5507/fot.2011.012
Dhiab RB, Ouada HB, Boussetta H, Franck F, Elabed A, Brouers M (2007) Growth, fluorescence, photosynthetic O2 production and pigment content of salt adapted cultures of Arthrospira (Spirulina) platensis. J Appl Phycol 19(4):293–301. https://doi.org/10.1007/s10811-006-9113-z
El-Din SMM (2017) Effect of copper and lead on growth and some metabolic activities of cyanobacterium Spirulina platensis (Nordstedt). Egypt J Bot 57(3):445–456. https://doi.org/10.21608/ejbo.2017.822.1055
Fraser JM, Tulk SE, Jeans JA, Campbell DA, Bibby TS, Cockshutt AM (2013) Photophysiological and photosynthetic complex changes during iron starvation in Synechocystis sp. PCC 6803 and Synechococcus elongatus PCC 7942. PLoS ONE 8(3):e59861. https://doi.org/10.1371/journal.pone.0059861
Galetović A, Seura F, Gallardo V, Graves R, Cortés J, Valdivia C, Núñez J, Tapia C, Neira I, Sanzana S, Gómez-Silva B (2020) Use of phycobiliproteins from Atacama cyanobacteria as food colorants in a dairy beverage prototype. Foods 9(2):244. https://doi.org/10.3390/FOODS9020244
Giraldez-Ruiz N, Mateo P, Bonilla I, Fernandez-Piñas F (1997) The relationship between intracellular pH, growth characteristics and calcium in the cyanobacterium Anabaena sp. strain PCC7120 exposed to low pH. New Phytol 137(4):599–605. https://doi.org/10.1046/j.1469-8137.1997.00864.x
Grigoryeva N, Chistyakova L (2019) Confocal laser scanning microscopy for spectroscopic studies of living photosynthetic cells. In: Color detection. IntechOpen. https://doi.org/10.5772/intechopen.84825
Hinners J, Hofmeister R, Hense I (2015) Modeling the role of pH on baltic sea cyanobacteria. Life 5(2):1204–1217. https://doi.org/10.3390/life5021204
Inoue-Kashino N, Kashino Y, Satoh K, Terashima I, Pakrasi HB (2005) PsbU provides a stable architecture for the oxygen-evolving system in cyanobacterial photosystem II. Biochemistry 44(36):12214–12228. https://doi.org/10.1021/bi047539k
Jangir MM, Chowdhury S, Bhagavatula V (2021) Differential response of photosynthetic apparatus towards alkaline pH treatment in NIES-39 and PCC 7345 strains of Arthrospira platensis. Int Microbiol 24(2):219–231. https://doi.org/10.1007/s10123-021-00160-6
Jin Q, Kirk MF (2018) pH as a primary control in environmental microbiology: 1. Thermodyn Perspect Front Environ Sci 6:21. https://doi.org/10.3389/FENVS.2018.00021
Katoch M, Mazmouz R, Chau R, Pearson LA, Pickford R, Neilan BA (2016) Heterologous production of cyanobacterial mycosporine-like amino acids mycosporine-ornithine and mycosporine-lysine in Escherichia coli. Appl Environ Microbiol 82(20):6167–6173. https://doi.org/10.1128/AEM.01632-16
Kaushik BD (1994) Algalization of rice in salt-affected soils. Ann Agric Res 14:105–106
Kenesi G, Shafik HM, Kovács AW, Herodek S, Présing M (2009) Effect of nitrogen forms on growth, cell composition and N2 fixation of Cylindrospermopsis raciborskii in phosphorus-limited chemostat cultures. Hydrobiologia 623(1):191–202. https://doi.org/10.1007/s10750-008-9657-9
Khan H, Laas A, Marcé R, Obrador B (2020) Major effects of alkalinity on the relationship between metabolism and dissolved inorganic carbon dynamics in lakes. Ecosystems 23(8):1566–1580. https://doi.org/10.1007/s10021-020-00488-6
Khazi MI, Demirel Z, Dalay MC (2018) Evaluation of growth and phycobiliprotein composition of cyanobacteria isolates cultivated in different nitrogen sources. J Appl Phycol 30(3):1513–1523. https://doi.org/10.1007/s10811-018-1398-1
Klanchui A, Cheevadhanarak S, Prommeenate P, Meechai A (2017) Exploring components of the CO2-concentrating mechanism in alkaliphilic cyanobacteria through genome-based analysis. Comput Struct Biotechnol J 15:340–350. https://doi.org/10.1016/j.csbj.2017.05.001
Krausfeldt LE, Farmer AT, Castro Gonzalez HF, Zepernick BN, Campagna SR, Wilhelm SW (2019) Urea Is both a carbon and nitrogen source for Microcystis aeruginosa: tracking 13C incorporation at bloom pH conditions. Front Microbiol 10:1064. https://doi.org/10.3389/fmicb.2019.01064
Lee H, Noh YJ, Hong SJ, Lee H, Kim DM, Cho BK, Lee CG, Choi HK (2021) Photosynthetic pigment production and metabolic and lipidomic alterations in the marine cyanobacteria Synechocystis sp. PCC 7338 under various salinity conditions. J Appl Phycol 33(1):197–209. https://doi.org/10.1007/s10811-020-02273-3
Leu JY, Lin TH, Selvamani MJP, Chen HC, Liang JZ, Pan KM (2013) Characterization of a novel thermophilic cyanobacterial strain from Taian hot springs in Taiwan for high CO2 mitigation and C-phycocyanin extraction. Process Biochem 48(1):41–48. https://doi.org/10.1016/J.PROCBIO.2012.09.019
Li P, Liu W, Gao K (2013) Effects of temperature, pH, and UV radiation on alkaline phosphatase activity in the terrestrial cyanobacterium Nostoc flagelliforme. J Appl Phycol 25(4):1031–1038. https://doi.org/10.1007/s10811-012-9936-8
Li Y, Lin Y, Loughlin PC, Chen M (2014) Optimization and effects of different culture conditions on growth of Halomicronema hongdechloris—a filamentous cyanobacterium containing chlorophyll f. Front Plant Sci 5:67. https://doi.org/10.3389/fpls.2014.00067
Mangan N, Brenner M (2014) Systems analysis of the CO2 concentrating mechanism in cyanobacteria. Elife 3:e02043. https://doi.org/10.7554/eLife.02043
Mareš J, Jek JH, Urajová P, Kopecký J, Hrouzek P (2014) A hybrid non-ribosomal peptide/polyketide synthetase containing fatty-acyl ligase (Faal) synthesizes the b-amino fatty acid lipopeptides puwainaphycins in the cyanobacterium cylindrospermum alatosporum. PLoS ONE 9(11):e111904. https://doi.org/10.1371/journal.pone.0111904
Marker AFH (1972) The use of acetone and methanol in the estimation of chlorophyll in the presence of phaeophytin. Freshw Biol 2(4):361–385. https://doi.org/10.1111/j.1365-2427.1972.tb00377.x
Miriam LM, Raj RE, Kings AJ, Visvanathan MA (2017) Identification and characterization of a novel biodiesel producing halophilic Aphanothece halophytica and its growth and lipid optimization in various media. Energy Convers Manag 141:93–100. https://doi.org/10.1016/j.enconman.2016.05.041
Müller C, Reuter W, Wehrmeyer W, Dau H, Senger H (1993) Adaptation of the photosynthetic apparatus of Anacystis nidulans to Irradiance and CO2-concentration. Botanica Acta 106(6):480–487. https://doi.org/10.1111/j.1438-8677.1993.tb00777.x
Nguyen BT, Rittmann BE (2015) Predicting dissolved inorganic carbon in photoautotrophic microalgae culture via the nitrogen source. Environ Sci Technol 49(16):9826–9831. https://doi.org/10.1021/acs.est.5b01727
Nygård CA, Dring MJ (2008) Influence of salinity, temperature, dissolved inorganic carbon and nutrient concentration on the photosynthesis and growth of Fucus vesiculosus from the Baltic and Irish Seas. Eur J Phycol 43(3):253–262. https://doi.org/10.1080/09670260802172627
Pathak J, Maurya PK, Singh SP, Häder DP, Sinha RP (2018) Cyanobacterial farming for environment friendly sustainable agriculture practices: innovations and perspectives. Front Environ Sci 6:7. https://doi.org/10.3389/fenvs.2018.00007
Pawlik-Skowrońska B, Kaczorowska R, Skowroński T (1997) The impact of inorganic tin on the planktonic cyanobacterium Synechocystis aquatilis: the effect of pH and humic acid. Environ Pollut 97(1–2):65–69. https://doi.org/10.1016/S0269-7491(97)00074-2
Pedersen O, Colmer TD, Sand-Jensen K (2013) Underwater photosynthesis of submerged plants—recent advances and methods. Front Plant Sci 4:140. https://doi.org/10.3389/fpls.2013.00140
Poza-Carrión C, Fernández-Valiente E, Piñas FF, Leganés F (2001) Acclimation of photosynthetic pigments and photosynthesis of the cyanobacterium Nostoc sp. strain UAM206 to combined fluctuations of irradiance, pH, and inorganic carbon availability. J Plant Physiol 158(11):1455–1461. https://doi.org/10.1078/0176-1617-00555
Ramírez M, Hernández-Mariné M, Mateoc P, Berrendero E, Roldán M (2011) Polyphasic approach and adaptative strategies of Nostoc cf. commune (Nostocales, Nostocaceae) growing on Mayan monument. Fottea. https://doi.org/10.5507/fot.2011.008
Sakshaug E, Bricaud A, Dandonneau Y, Falkowski PG, Kiefer DA, Legendre L, Morel A, Parslow J, Takahashi M (1997) Parameters of photosynthesis: definitions, theory and interpretation of results. J Plankton Res 19(11):1637–1670. https://doi.org/10.1093/plankt/19.11.1637
Schmitt FJ, Campbell ZY, Moldenhauer M, Friedrich T (2020) Light-induced phycobilisome dynamics in Halomicronema hongdechloris. J Photochem Photobiol A 403:112838. https://doi.org/10.1016/J.JPHOTOCHEM.2020.112838
Schulze K, López DA, Tillich UM, Frohme M (2011) A simple viability analysis for unicellular cyanobacteria using a new autofluorescence assay, automated microscopy, and ImageJ. BMC Biotechnol. https://doi.org/10.1186/1472-6750-11-118
Shokravi S, Bahavar N (2021a) Growth and photosynthesis acclimated response of the cyanobacterium Fischerella sp. FS 18 exposed to extreme conditions: alkaline pH, limited irradiance, and carbon dioxide concentration. Extremophiles 25(5):493–500. https://doi.org/10.1007/s00792-021-01244-x
Shokravi S, Bahavar N (2021b) Effects of chromium (VI) at extreme alkaline condition (pH 11) on the survival, growth, photosystems and phycobilisome operation of the cyanobacterium Synechocystis sp. strain FS 78. J Appl Phycol 33(5):2909–2919. https://doi.org/10.1007/s10811-021-02521-0
Shokravi S, Soltani N (2011) Acclimation of the hapalosiphon sp. (Cyanoprokaryota) to combination effects of dissolved inorganic carbon and pH at extremely limited irradiance. Int J Algae. https://doi.org/10.1615/InterJAlgae.v13.i4.60
Siahbalaei R, Afsharzadeh S, Shokravi S (2011) New Records of nostocalean cyanobacteria from rice fields in the Golestan Province in North-East of Iran. Progr Biol Sci 1(2):50–55. https://doi.org/10.22059/pbs.2011.24290
Singh H (2018) Desiccation and radiation stress tolerance in cyanobacteria. J Basic Microbiol 58(10):813–826. https://doi.org/10.1002/jobm.201800216
Singh DP, Khare P, Bisen PS (1989) Effect of Ni2+, Hg2+ and Cu2+ on growth, oxygen evolution and photosynthetic electron transport in Cylindrospermum IU 942. J Plant Physiol 134(4):406–412. https://doi.org/10.1016/S0176-1617(89)80003-3
Singh V, Pandey KD, Mesapogu S, Singh DV (2015) Influence of NaCl on photosynthesis and nitrogen metabolism of cyanobacterium Nostoc calcicola. Appl Biochem Microbiol. https://doi.org/10.1134/S0003683815060149
Soltani N, Khavari-Nejad RA, Yazdi MT, Shokravi S, Fernández-Valiente E (2006) Variation of nitrogenase activity, photosynthesis and pigmentation of the cyanobacterium Fischerella ambigua strain FS18 under different irradiance and pH values. World J Microbiol Biotechnol. https://doi.org/10.1007/s11274-005-9073-5
Srivastava AK, Bhargava P, Kumar A, Rai LC, Neilan BA (2009) Molecular characterization and the effect of salinity on cyanobacterial diversity in the rice fields of Eastern Uttar Pradesh, India. Saline Syst 5(1):1–17. https://doi.org/10.1186/1746-1448-5-4
Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35(2):171–205. https://doi.org/10.1128/mmbr.35.2.171-205
Sugiura K, Itoh S (2012) Single-cell confocal spectrometry of a filamentous cyanobacterium nostoc at room and cryogenic temperature. Diversity and differentiation of pigment systems in 311 cells. Plant Cell Physiol. https://doi.org/10.1093/pcp/pcs093
Summerfield TC, Crawford TS, Young RD, Chua JPS, MacDonald RL, Sherman LA, Eaton-Rye JJ (2013) Environmental pH affects photoautotrophic growth of synechocystis sp. PCC 6803 strains carrying mutations in the lumenal proteins of PSII. Plant Cell Physiol. https://doi.org/10.1093/pcp/pct036
Tang EPY, Vincent WF (1999) Strategies of thermal adaptation by high-latitude cyanobacteria. New Phytol. https://doi.org/10.1046/j.1469-8137.1999.00385.x
Tiwari S, Mohanty P (1996) Cobalt induced changes in photosystem activity in Synechocystis PCC 6803: alterations in energy distribution and stoichiometry. Photosynth Res 50(3):243–256. https://doi.org/10.1007/BF00033123
Touloupakis E, Cicchi B, Benavides AM, Torzillo G (2016) Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.). Appl Microbiol Biotechnol 100(3):1333–1341. https://doi.org/10.1007/s00253-015-7024-0
Valiente EF, Leganes F (1990) Regulatory effect of pH and incident irradiance on the levels of nitrogenase activity in the cyanobacterium Nostoc UAM 205. J Plant Physiol 135(5):623–627. https://doi.org/10.1016/S0176-1617(11)80647-4
Vermaas WFJ, Timlin JA, Jones HDT, Sinclair MB, Nieman LT, Hamad SW, Melgaard DK, Haaland DM (2008) In vivo hyperspectral confocal fluorescence imaging to determine pigment localization and distribution in cyanobacterial cells. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.0708090105
Vonshak A, Torzillo G (2007) Environmental stress physiology. Handb Microalgal Cult. https://doi.org/10.1002/9780470995280.CH4
Wolf E, Schüßler A (2005) Phycobiliprotein fluorescence of Nostoc punctiforme changes during the life cycle and chromatic adaptation: Characterization by spectral confocal laser scanning microscopy and spectral unmixing. Plant Cell Environ 28(4):480–491. https://doi.org/10.1111/j.1365-3040.2005.01290.x
Ye C, Gao K (2004) Photosynthetic response to salt of aquatic-living colonies of the terrestrial cyanobacterium Nostoc flagelliforme. J Appl Phycol 16(6):477–481. https://doi.org/10.1007/s10811-004-5509-9
Ying L, Huang X, Huang B, Xie J, Zhao J, Zhao XS (2002) Fluorescence emission and absorption spectra of single Anabaena sp. strain PCC7120 cells. Photochem Photobiol 76(3):310–313. https://doi.org/10.1562/0031-8655(2002)0760310FEAASO2.0.CO2
Zorz JK, Allanach JR, Murphy CD, Roodvoets MS, Campbell DA, Cockshutt AM (2015) The RUBISCO to photosystem II ratio limits the maximum photosynthetic rate in picocyanobacteria. Life 5(1):403–417. https://doi.org/10.3390/life5010403
Acknowledgements
The authors would like to appreciate the kind collaboration of Professor Eduardo Fernandez Valiente (Universidad Autónoma de Madrid -UAM) and Professor Neda Soltani (Shahid Beheshti University, Iran). This work was supported in part by Doctor Enrique Llobet Lleó to assist the world microbial community.
Funding
Open Access Funding provided by Universitat Autonoma de Barcelona.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I and Shadman Shokravi have no conflict of interest to declare.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bahavar, N., Shokravi, S. Acclimation response and ability of growth and photosynthesis of terrestrial cyanobacterium Cylindrospermum sp. strain FS 64 under combined environmental factors. Arch Microbiol 204, 165 (2022). https://doi.org/10.1007/s00203-022-02772-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02772-6