Abstract
A novel thermophilic Gram staining positive strain Rx1 was isolated from hot springs in Baoshan of Yunnan Province, China. The strain was characterized as a hemicellulose-decomposing obligate anaerobe bacterium that is rod-shaped (diameter: 0.5–0.7 μm; length: 2.0–6.7 μm), spore-forming, and motile. Its growth temperature range is 38–68 °C (optimum 50–55 °C) and pH range is 4.5–8.0 (optimum 7.0). The maximum tolerance concentration of NaCl was 3 %. Rx1 converted thiosulfate to elemental sulfur and reduced sulfite to hydrogen sulfide. The bacterium grew by utilizing xylan and starch, as well as a wide range of monosaccharide and polysaccharides, including glucose and xylose. The main products of fermentation were ethanol, lactate, acetate, CO2, and H2. The maximum xylanase activity in the culture supernatant after 30 h of incubation at 55 °C was 16.2 U/ml. Rx1 DNA G + C content was 36 mol %. 16S rRNA gene sequence analysis indicated that strain Rx1 belonged to the genus Thermoanaerobacterium of the family ‘Thermoanaerobacteriaceae’ (Firmicutes), with Thermoanaerobacterium aciditolerans 761–119 (99.2 % 16S rRNA gene sequence similarity) being its closest relative. DNA–DNA hybridization between Rx1 and T. aciditolerans 761–119 showed 36 % relatedness. Based on its physiological and biochemical tests and DNA–DNA hybridization analyses, the isolate is considered to represent a novel species in the genus Thermoanaerobacterium, for which the name Thermoanaerobacterium calidifontis sp. nov. is proposed, with the type strain is Rx1 (=JCM 18270 = CCTCC M 2011109).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The biotechnological potential and evolutionary significance of thermophiles has led to intensive research focused on anaerobic, saccharolytic, and thermophilic bacteria, which are members of the genera Thermoanaerobacter, Thermoanaerobacterium, and Clostridium (Xue et al. 2001). Within the genus Thermoanaerobacterium, eight species have been isolated, and more recently the taxonomic relationships between some of these species have been better defined (Romano et al. 2010). Most studies of eubacterial thermophilic anaerobes have focused on the saccharolytic bacteria, which form ethanol and lactate and are promising tools for creating alternative fuels from plant biomass (Shaw et al. 2008; Patel et al. 2006). Moreover, their ethanol production capacity involving utilization of glucose and the other hexose transformed from cellulose materials has been the major focus of those studies. Since only a few of the natural anaerobic thermophilic microorganisms identified to date are capable of efficiently fermenting xylose and the other pentose transformed from hemicellulose materials, we aimed to isolate novel thermophilic anaerobic hemicellulose-decomposing bacteria from hot springs sample.
The Baoshan region is situated in the southwest of Yunnan Province and encompasses a large number of natural hot springs. Water and sediment samples were collected from the region’s hot springs and measured temperature (50–70 °C) and pH (6.0–7.5). Using oat spelt xylan as substrate, we isolated a Gram staining positive, obligately anaerobic, thermophilic bacterium (strain Rx1).
In this paper, we describe the isolation and characterization of the ethanol-producing strain Rx1. The physiological, biochemical, and phenotypic features of Rx1 were determined, and the results of DNA–DNA relatedness studies indicated that Rx1 is a new member of the genus Thermoanaerobacterium. The name Themoanaerobacterium calidifontis is proposed.
Materials and methods
Strains and culture conditions
Thermoanaerobacterium aciditolerans DSM 16487T and Thermoanaerobacterium saccharolyticum DSM7060T were purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ; Braunschweig, Germany) and were cultured in modified medium 640 at 55 °C.
All culturing of Rx1 was performed in modified DSMZ medium 640, containing (per liter distilled water) 0.9 g NH4Cl, 0.9 g NaCl, 0.4 g MgCl2 × 6 H2O, 0.75 g KH2PO4, 1.5 g K2HPO4, 2.5 mg FeCl3 × 6 H2O, 0.75 g cysteine-HCl × H2O, 2 g tryptone, 1 g yeast extract, 1 ml trace element solution SL-10 (see medium 320, DSMZ), 0.5 mg resazurin, 5 ml vitamin solution (see medium 141, DSMZ, filter-sterilized), 10.0 g substrate. After adjusting pH to 7.0, the medium was prepared anaerobically under 100 % N2 and dispensed 5 ml portions into 15 ml Hungate tubes. Finally, the medium solutions were autoclaved.
Initial enrichment was carried out at 60 °C by inoculating the samples of mixed sediment and water into 250 ml anaerobic reagent bottles (50 ml modified medium 640). Oat spelt xylan was added as substrate, and the culture incubated until visible growth was observed. The ethanol-producing cultures were isolated through several serial liquid dilutions and applied to the anaerobic phytagel (1.2 %, w/v) shake-roll tube technique (Ljungdahl and Wiegel 1986) with xylose as substrate. Pure strains were stored as liquid cultures under anaerobic conditions at 4 and −80 °C. One of the isolated strains was strain Rx1 for further characterization.
Morphological, physiological, and biochemical analysis
Gram reaction was determined using a Gram staining kit (Guangdong Huankai Microbial Sci. & Tech Co., Ltd. Guangdong, China). Cell morphology was examined by phase-contrast microscopy (Leica, Germany). Bacteria tolerance of NaCl (0–5 %, w/v), temperature (from 35 to 70 °C), pH (from 3.5 to 8.5), and substrates (complete list in Table 1) was determined by growing on the modified 640 medium for four days and measuring the optical density (OD) at 600 nm using a 4802 UV/VIS double-beam spectrophotometer (Unico, Dayton, NJ, USA). Metabolite products were measured by high-performance liquid chromatography (HPLC) and gas chromatography (GC-SC2), as previously described (Shaw et al. 2008; Romano et al. 2010; Ren et al. 2008). Ethanol tolerance was determined by supplementing the modified 640 medium with ethanol (from 0 to 5.0 %, v/v) after autoclaving. The ability of Rx1 to convert thiosulfate, sulfate, sulfite, and elemental sulfur was assessed by adding thiosulfate (7 g/l), sulfate (2 g/l), sulfite (0.76 g/l), or elemental sulfur (2 g/l), respectively, to the modified medium 640 (Kublanov et al. 2007; Liu et al. 1996). All the tests were performed in triplicate. Phase-contrast microscopy was used to visualize the sulfur globules formation (Lee et al. 2007). The gas from the headspace (10 ml) culture of Rx1 was transferred via sterile syringe into a test tube containing 5 ml of 50 g/l copper sulfate solution, and the formation a black precipitate of copper sulfide was observed. At the same time, one control sample was used without gas injected in the test tube, and another control sample was used with gas injected from the headspace of Rx1 cultures, but without added sulfate, sulfite, or elemental sulfur in the modified medium 640.
Enzymatic hydrolysis
A 500 ml anaerobic reagent bottle containing 250 ml of modified 640 medium was inoculated with 2.5 ml of an overnight culture of Rx1 in the exponential growth phase. Fermentation was carried out at 55 °C with 10 g/l of oat spelt xylan as substrate. Aliquots of the fermentation liquid were collected at different time points and were centrifuged (12,000 rpm, 5 min) to remove cells. The supernatant (enzyme solution) was then analyzed as described below.
Xylanase activity was determined in triplicate reactions using the dinitrosalicylic acid (DNS) method (Bailey et al. 1992). The initial reaction mixture consisted of 0.5 ml of 1 mM oat spelt xylan in water and 0.5 ml of enzyme solution. After incubation at 55 °C for 10 min, the DNS reagent was added, and the mixture was boiled at 100 °C for 5 min. After cooling, water was added to bring the volume to 20 ml, and the amount of reduced sugar released from the xylan substrate was measured as OD540. The control reaction lacked the enzyme solution. Measurements of a series of xylose dilutions were used as standards to calculate the quantity of reduced sugar. One unit of xylanase activity was defined as the activity that released 1 μmol of xylose in 1 min under the assay conditions.
Polyacrylamide gel electrophoresis (PAGE) of soluble proteins
SDS-PAGE of whole cell protein extracts is used widely in bacterial taxonomy and is suitable for grouping strains at the species level (Bandyopadhyay et al. 2013; Dicks et al. 1990). For total soluble cell protein analysis, 10 ml aliquots of late exponential phase cultures grown at 55 °C in the modified medium (pH 6.5) containing 1.0 % glucose as the substrate was harvested by centrifugation. The total cellular protein was extracted as described previously (Liu et al. 1996), and the protein concentration was determined by the Bradford method (Bradford 1976). Samples containing 50 μg of protein from each strain were analyzed by SDS-PAGE, as previously described (Laemmli 1970).
16S rRNA gene sequencing and phylogenetic analysis
Bacterial 16S rRNA was amplified by PCR using the previously described conditions (Rivas et al. 2009) and the following universal primers: reverse 1541R, 5′-TGYGGNTGGATCACCTCCTT-3′ (Escherichia coli positions 1,509–1,522) and forward 8F, 5′-AGAGTTTGATCTGGCTCAG-3′ (E. coli positions 8–27). Sequence comparison of the 16S rRNA gene was carried out using the BLAST program (http://www.ncbi.nlm.nih.gov/blast) and the global alignment algorithm of the EzTaxon server (http://www.eztaxon.org/; Chun et al. 2007). After multiple sequence alignment by ClustalX (Thompson et al. 1997), a phylogenetic tree was constructed by the neighbor-joining and minimum evolution methods using Molecular Evolutionary Genetics Analysis (MEGA) software, version 5.0 (Tamura et al. 2011) and evaluated by bootstrap resampling with 1,000 replicates.
Determination of G + C and DNA–DNA hybridization
Genomic DNA was extracted, and G + C content was evaluated in triplicate samples by HPLC using the method described by Murray and Thompson (1980). Briefly, purified DNA samples were mixed with 45 % perchloric acid (DNA: 45 % perchloric acid = 10 μg:1 μl) and boiled at 98 °C for 2 h. Supernatants were harvested by centrifugation (5,000g, 10 min, 4 °C) and diluted 50-fold using distilled water for HPLC analysis (Mesbah et al. 1989).
The DNA–DNA hybridization technique has been especially successful in resolving taxonomic relationships among closely related organisms (at the species level and below) (Lee et al. 1993), Here, this technique was carried out according to the spectrophotometric method (Ley et al. 1970; Huss et al. 1983; Jahnke 1992), using a UV-1700 spectrophotometer (Shimadzu, Kyoto, Japan) equipped with a DCW-2008 thermo bath (Yuan et al. 2008). G + C content and DNA–DNA hybridization assays were performed in triplicate.
Results
Isolation and characterization of a novel anaerobic, thermophilic, hemicellulose-decomposing bacteria
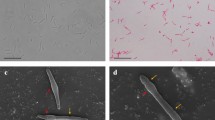
An anaerobic, thermophilic, ethanol-producing strain (Rx1) was obtained from samples of mixed sediment and water of hot springs. Colonies of Rx1 grown within the phytagel shake-roll tubes appeared as white, circular, 0.5–1 mm in diameter, and slightly convex (colonies were adhered to the inner surface of the phytagel shake-roll tubes). Analysis of the single cells indicated that the strain was Gram staining positive, motile, rod-shaped, 0.5–0.7 μm in diameter, and 2.0–6.7 μm in length (Table 1). The average (mean of n = 15) length of the individual organisms was longer in the stationary phase than in the exponential growth phase. The formation of terminal spores was observed during the late exponential and early stationary phase (Fig. 1). Approximately 6.0 % of the cells sporulated in liquid media, and the spores were oval, 1.5–1.7 μm in length and 1.0–1.5 μm in diameter. The length of the end of the mother cells ranged between 5.9 and 7.6 μm, so that they were longer than normal cells. The strain did not grow in oxidized medium, as evidenced by the pink color of resazurin. The strain had a temperature growth range from 38 °C to 68 °C, with the optimal temperature being between 50 and 55 °C. For up to 96 h, no growth was observed at temperatures ≤37 °C or ≥70 °C. The pH range for growth at 55 °C was between pH 4.5 and 8.0, with the optimum pH being 7.0. For up to 96 h, no growth was observed at pH ≤ 4.3 or ≥8.2. The growth curve of Rx1 under optimal growth condition with 5 g/l xylose substrate is shown in Fig. 3; the doubling time under the optimal growth condition was approximately 50 min. Growth of Rx1 occurred at NaCl concentrations ranging from 0 to 3 %, but no growth was observed in 4 % NaCl.
Rx1 was able to grow in the presence of the following polysaccharide substrates: xylan, starch, and pectin. It also was able to utilize various monosaccharides and disaccharides, including glucose, xylose, fructose, mannose, mannitol, galactose, maltose, lactose, cellobiose, arabinose, and pyruvate (filter-sterilized). However, no growth was observed in the presence of sucrose, xylitol, sorbitol, cellulose, glycerol, or ethanol (Table 1). The fermentation products from xylan, starch, cellobiose, xylose, and glucose substrates were mainly ethanol, acetate, lactate, CO2, and H2 (production of H2 and CO2 was detected by gas chromatography), respectively. Under optimal conditions, when Rx1 was grown on 5 g/l of glucose for 48 h, the fermentation products were ethanol (29.3 mM), acetate (13.1 mM), lactate (16.2 mM), H2 (38.1 mM), and CO2 (8.6 mM). Ethanol yield reached 58 % of the theoretical yield (we assumed that all of consumed glucose/xylose was converted into ethanol). In optimal conditions, when Rx1 was grown on 5 g/l of xylose for 48 h, the amounts of ethanol, acetate, lactate, H2 and CO2 were 45.1, 1.3, 10.5, 29.7, and 8.8 mM, respectively. Ethanol yield reached 81 % of the theoretical yield. When Rx1 was grown on 5 g/l of xylan for 48 h, the amounts of ethanol, acetate, and lactate were 25.2, 0.8, and 7.3 mM, respectively. These results indicated that Rx1 was able to produce ethanol from the majority of hexose and pentose, even polysaccharides; the ethanol yields were 1.0–4.3 and 2.2–34.7 times those of lactate and acetate, respectively. However, ethanol yields of most of the known Thermoanaerobacterium species were lower or equal to those for lactate and acetate (Romano et al. 2010; Kublanov et al. 2007; Liu et al. 1996). Therefore, Rx1 is considered as potentially useful strains for converting biomass to produce ethanol.
Rx1 was able to grow in the presence of 3 % (v/v) ethanol. Moreover, addition of thiosulfate (7 g/l) to the modified 640 medium significantly stimulated growth of Rx1. Rx1 was found to convert thiosulfate to sulfur globules (S0), which were detected both inside the cells and in the medium (Fig. 2), but the ethanol yield of Rx1 decreased under this condition by 22 %. Rx1 also grew well in the presence of sulfate, elemental sulfur and sulfite, and sulfite was reduced to hydrogen sulfide, but neither sulfate nor elemental sulfur was reduced (Fig. S1); however, the bacterial growth and ethanol production capability were not remarkably influenced by these medium conditions (Fig. 3).
Thermoanaerobacterium calidifontis Rx1 grown in modified 640 medium containing 1 % xylose and 7 g/l Na2S2O3 at 55 °C. The cells of strain Rx1 are shown during the late exponential or early stationary phase. Note that the phase-bright sulfur globules accumulated in the medium and in the cells. Bar 10 μm
Rx1 grew on xylan, and xylanase activities were detected in culture supernatants after 8 h. The highest enzyme activity was 16.2 U/ml after culture for 30 h at 55 °C (Fig. 3). Xylanase activity in culture supernatants were compared with those detected from T. thermosulfurigenes (0.023 U/ml), T. xylanolyticum (0.067 U/ml), and T. saccharolyticum (0.108 U/ml) strains of the Thermoanaerobacterium genus, and found to be up to 703, 241 and 150 times higher (Lee et al. 1993). Xylanase may be a thermostable enzyme, giving it potential in applications for the ethanol-producing industry. The properties, production, and applications of xylanase need to be further researched.
Protein profile
Strain Rx1 showed distinct protein pattern from those of its closest related reference strains (Fig. 4), indicating that strain Rx1 is different from the other two species of the Thermoanaerobacterium genera and supporting its identification as a novel species.
Phylogenetic relationships
BLAST search of the 1,473 nucleotide sequence of the Rx1 16S rRNA gene revealed that this novel strain was a member of genus Thermoanaerobacterium of the family Thermoanaerobacteriaceae, which belongs to the order Thermoanaerobacteriales of the class Clostridia in the phylum Firmicutes (Garrity et al. 2003). Rx1 showed the highest identity with T. aciditolerans 761–119T (99.2 %) (Kublanov et al. 2007). The phylogenetic tree indicated that stain Rx1 was a member of the genus Thermoanaerobacterium (Fig. 5). Similar tree topologies were also found by the tree maximum evolution method (data not shown). DNA–DNA hybridization between strain Rx1 and T. aciditolerans 761–119T showed relatedness of only 36 %. The DNA G + C content of strain Rx1 was 36 mol %, which was higher than that of T. aciditolerans 761–119T (34 ± 0.5 mol %).
Phylogenetic tree based on 16S rRNA gene sequences, showing the position of isolate T. calidifontis Rx1 in the genus Thermoanaerobacterium. The tree was constructed by neighbor-joining method with 1,000 replicates of bootstrapping. GenBank accession numbers are given in parentheses. Bootstraps are the confidence values (expressed as percentages) obtained from 1,000 replications. Bar 5 nucleotide substitutions per 100 nucleotides
Discussion
In this paper, we describe a new thermophilic anaerobic bacteria strain, Rx1, which was isolated from hot springs in Baoshan of Yunnan Province, China. Strain Rx1 showed the most sequence similarity (99.2 %) with T. aciditolerans. However, despite several phenotypic similarities, strain Rx1 differs from T. aciditolerans. As reported in Table 1, the pH range of Rx1 (4.5–8.0) was different from that of both T. aciditolerans (Kublanov et al. 2007) and T. aotearoense (Liu et al. 1996) (3.2–7.1 and 3.8–6.8, respectively). The optimum pH of Rx1 (7.0) was also higher than T. aciditolerans (5.7) and T. aotearoense (5.2). Rx1 could utilize pyruvate, but not sorbitol or sucrose, the opposite of T. aciditolerans. In addition, Rx1 has a high yield xylanase and was able to ferment a series of carbohydrates, which suggests that it may be applied in the ethanol production process.
On the basis of the above distinctive physiological and biochemical properties, G + C content and DNA–DNA hybridization values, the isolate Rx1 appears to represent a new species of the genus Thermoanaerobacterium. As such, the name Thermoanaerobacterium calidifontis sp. nov., is proposed.
Description of Thermoanaerobacterium calidifontis sp. nov.
Thermoanaerobacterium calidifontis: ca.li.di.fon’tis. L. adj. calidus, hot; L. n. fons fontis, spring, fountain; N.L. gen. n. calidifontis, of a hot spring.
Cells are Gram staining positive, rod-shaped, motile, an average diameter of 0.5–0.7 μm and length of 2.0–6.7 μm, obligate anaerobe, hemicellulose-decomposing bacteria. Colonies are circular, 0.5–1 mm in diameter, white, and slightly convex. Oval terminal spores were formed in the late exponential to early stationary phases of growth, and the spores were oval, 1.5–1.7 μm in length and 1.0–1.5 μm in diameter. The organism is a moderate thermophile that grows between 38 and 68 °C (no growth at ≤37 °C or ≥70 °C), with the optimum temperature between 50 and 55 °C, at pH between 4.5 and 8.0 (no growth at pH ≤ 4.3 or ≥8.2), with the optimum pH of 7.0, and low salinity (NaCl range 0–3 %). The bacteria grow by fermentation of xylan, starch, pectin, mannitol, glucose, xylose, fructose, galactose, maltose, lactose, cellobiose, mannose, arabinose, and pyruvate. Fermentation end products are ethanol, lactate, acetate, H2, and CO2. Under optimal growth conditions, the Rx1 strain fermented xylose and glucose for 48 h and produced ethanol yields that were 81 and 58 % of the theoretical yields, respectively. The bacteria did not utilize sucrose, xylitol, sorbitol, cellulose, glycerol, or ethanol. The bacteria were able to convert thiosulfate to elemental sulfur, which accumulated both in the cells and in the culture medium. Sulfite was reduced to hydrogen sulfide. The genomic DNA G + C content (measured by HPLC) of strain Rx1 was 36 mol %. The type strain Rx1 (= JCM 18270 = CCTCC M 2011109) was isolated from a hot spring in Baoshan, Yunnan Province, China.
References
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270
Bandyopadhyay S, Schumann P, Das SK (2013) Pannonibacter indica sp. nov., a highly arsenate-tolerant bacterium isolated from a hot spring in India. Arch Microbiol 195:1–8
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, Lim YW (2007) EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 57:2259–2261
Dicks LMT, Van Vuuren HJJ, Dellaglio F (1990) Taxonomy of Leuconostoc species, particularly Leuconostoc oenos, as revealed by numerical analysis of total soluble cell protein patterns, DNA base compositions, and DNA–DNA hybridization. Int J Syst Bacteriol 40:83–91
Garrity GM, Bell JA, Lilburn TG (2003) Taxonomic outline of the procaryotes. Bergey’s manual of systematic bacteriology, 2nd edn. Release 4:1–397
Huss VAR, Festl H, Schleifer KH (1983) Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol 4:184–192
Jahnke KD (1992) BASIC computer program for evaluation of spectroscopic DNA renaturation data from GILFORD SYSTEM 2600 spectrophotometer on a PC/XT/AT type personal computer. J Microbiol Meth 15:61–73
Kublanov IV, Prokofeva MI, Kostrikina NA, Kolganova TV, Tourova TP, Wiegel J, Bonch-Osmolovskaya EA (2007) Thermoanaerobacterium aciditolerans sp. nov., a moderate thermoacidophile from a Kamchatka hot spring. Int J Syst Evol Microbiol 57:260–264
Laemmli UK (1970) Cleavage of structure proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680–685
Lee YE, Jain MK, Lee C, Lowe SE, Zeikus JG (1993) Taxonomic distinction of saccharolytic thermophilic anaerobes: description of Thermoanaerobacterium xylanolyticum gen. nov., sp. nov., and Thermoanaerobacterium saccharolyticum gen. nov., sp. nov.; reclassification of Thermoanaerobium brockii, Clostridium thermosulfurogenes, and Clostridium thermohydrosulfuricum E100-69 as Thermoanaerobacter brockii comb. nov., Thermoanaerobacterium thermosulfurigenes comb. nov., and Thermoanaerobacter thermohydrosulfuricus comb. nov., respectively; and transfer of Clostridium thermohydrosulfuricum 39E to Thermoanaerobacter ethanolicus. Int J Syst Bacteriol 43:41–51
Lee YJ, Prange A, Lichtenberg H, Rohde M, Dashti M, Wiegel J (2007) In situ analysis of sulfur species in sulfur globules produced from thiosulfate by Thermoanaerobacter sulfurigignens and Thermoanaerobacterium thermosulfurigenes. J Bacteriol 189:7525–7529
Ley JD, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem 12:133–142
Liu SY, Rainey FA, Morgan HW, Mayer F, Wiegel J (1996) Thermoanaerobacterium aotearoense sp. nov., a slightly acidophilic, anaerobic thermophile isolated from various hot springs in New Zealand, and emendation of the genus Thermoanaerobacterium. Int J Syst Bacteriol 46:388–396
Ljungdahl LG, Wiegel J (1986) Working with anaerobic bacteria. In manual of industrial microbiology and miotechnology, Washington, pp 84–96
Mesbah M, Premachandran U, Whitman WB (1989) Precise measurement of the G + C content of deoxyribonucleic acid by high-performance liquid chromatography. Int J Syst Bacteriol 39:159–167
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326
Patel MA, Ou MS, Harbrucker R, Aldrich HC, Buszko ML, Ingram LO, Shanmugam KT (2006) Isolation and characterization of acid-tolerant, thermophilic bacteria for effective fermentation of biomass-derived sugars to lactic acid. Appl Environ Microbiol 72:3228–3235
Ren NQ, Cao GL, Wang AJ, Lee DJ, Guo WQ, Zhu YH (2008) Dark fermentation of xylose and glucose mix using isolated Thermoanaerobacterium thermosaccharolyticum W16. Int J Hydrogen Energ 33:6124–6132
Rivas R, García-Fraile P, Mateos PF, Martinez-Molina E, Velázquez E (2009) Phylogenetic diversity of fast-growing bacteria isolated from superficial water of lake martel, a saline subterranean lake in mallorca island (Spain) formed by filtration from the mediterranean sea through underground rocks. Adv Stud Biol 1:333–344
Romano I, Dipasquale L, Orlando P, Lama L, d’Ippolito G, Pascual J, Gambacorta A (2010) Thermoanaerobacterium thermostercus sp. nov., a new anaerobic thermophilic hydrogen-producing bacterium from buffalo-dung. Extremophiles 14:233–240
Shaw AJ, Podkaminer KK, Desai SG, Bardsley JS, Rogers Stephen R, Thorne PG, Hogsett DA, Lynd LR (2008) Metabolic engineering of a thermophilic bacterium to produce ethanol at high yield. PNAS 105:13769–13774
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Bio Evol 28:2731–2739
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Xue YF, Xu Y, Liu Y, Ma YH, Zhou PJ (2001) Thermoanaerobacter tengcongensis sp. nov., a novel anaerobic, saccharolytic, thermophilic bacterium isolated from a hot spring in Tengcong, China. Int J Syst Evol Microbiol 51:1335–1341
Yuan LJ, Zhang YQ, Guan Y, Wei YZ, Li QP, Yu LY, Li WJ, Zhang YQ (2008) Saccharopolyspora antimicrobica sp. nov., an actinomycete from soil. Int J Syst Bacteriol 58:1180–1185
Acknowledgments
The authors are very grateful to Dr. Jean Euzéby for helping with the new strain’s nomenclature and Dr. Wenjun Li and Lingling Yang for providing use of experimental instrumentation and for their assistance in some of the laboratory procedures. The Natural Science Foundation of Yunnan Province (Project No. 2009CD038) provided funding for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Harald Huber.
The GenBank/EMBL/DDBJ accession number for the 16S rRNA gene sequence of strain Rx1 is AB544080.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Shang, Sm., Qian, L., Zhang, X. et al. Themoanaerobacterium calidifontis sp. nov., a novel anaerobic, thermophilic, ethanol-producing bacterium from hot springs in China. Arch Microbiol 195, 439–445 (2013). https://doi.org/10.1007/s00203-013-0895-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-013-0895-5