Abstract
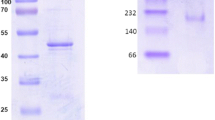
Pseudomonas putida CSV86 utilizes benzyl alcohol via catechol and methylnaphthalenes through detoxification pathway via hydroxymethylnaphthalenes and naphthaldehydes. Based on metabolic studies, benzyl alcohol dehydrogenase (BADH) and benzaldehyde dehydrogenase (BZDH) were hypothesized to be involved in the detoxification pathway. BADH and BZDH were purified to apparent homogeneity and were (1) homodimers with subunit molecular mass of 38 and 57 kDa, respectively, (2) NAD+ dependent, (3) broad substrate specific accepting mono- and di-aromatic alcohols and aldehydes but not aliphatic compounds, and (4) BADH contained iron and magnesium, while BZDH contained magnesium. BADH in the forward reaction converted alcohol to aldehyde and required NAD+, while in the reverse reaction it reduced aldehyde to alcohol in NADH-dependent manner. BZDH showed low K m value for benzaldehyde as compared to BADH reverse reaction. Chemical cross-linking studies revealed that BADH and BZDH do not form multi-enzyme complex. Thus, the conversion of aromatic alcohol to acid is due to low K m and high catalytic efficiency of BZDH. Phylogenetic analysis revealed that BADH is a novel enzyme and diverged during the evolution to gain the ability to utilize mono- and di-aromatic compounds. The wide substrate specificity of these enzymes enables strain to detoxify methylnaphthalenes to naphthoic acids efficiently.




Similar content being viewed by others
References
Basu A, Dixit SS, Phale PS (2003) Metabolism of benzyl alcohol via catechol ortho-pathway in methylnaphthalene-degrading Pseudomonas putida CSV86. Appl Microbiol Biotechnol 62:579–585
Biegert T, Altenschmidt U, Eckerskorn C, Fuchs G (1995) Purification and properties of benzyl alcohol dehydrogenase from a denitrifying Thauera sp. Arch Microbiol 163:418–423
Chalmers RM, Scott AJ, Fewson CA (1990) Purification of the benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase encoded by the TOL plasmid pWW53 of Pseudomonas putida MT53 and their preliminary comparison with benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase I and II from Acinetobacter calcoaceticus. J Gen Microbiol 136:637–643
Chalmers RM, Keen JN, Fewson CA (1991) Comparison of benzyl alcohol dehydrogenases and benzaldehyde dehydrogenases from the benzyl alcohol and mandelate pathways in Acinetobacter calcoaceticus and from the TOL-plasmid-encoded toluene pathway in Pseudomonas putida. N-terminal amino acid sequences, amino acid compositions and immunological cross-reactions. Biochem J 273(Pt 1):99–107
Dickinson FM, Berrieman S (1977) The reactions of 1,10-phenanthroline with yeast alcohol dehydrogenase. Biochem J 167:237–244
Drum DE, Harrison JH, Li TK, Bethune JL, Vallee BL (1967) Structural and functional zinc in horse liver alcohol dehydrogenase. Proc Natl Acad Sci U S A 57:1434–1440
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Keat MJ, Hopper DJ (1976) Aromatic-alcohol dehydrogenases from Pseudomonas putida N.C.I.B. 9869. Biochem Soc Trans 4:494–495
Keat MJ, Hopper DJ (1978) The aromatic alcohol dehydrogenases in Pseudomonas putida N.C.I.B. 9869 grown on 3, 5-xylenol and p-cresol. Biochem J 175:659–667
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Luykx DM, Duine JA, de Vries S (1998) Molybdopterin radical in bacterial aldehyde dehydrogenases. Biochemistry 37:11366–11375
MacKintosh RW, Fewson CA (1987) Microbial aromatic alcohol and aldehyde dehydrogenases. Prog Clin Biol Res 232:259–273
MacKintosh RW, Fewson CA (1988a) Benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase II from Acinetobacter calcoaceticus. Purification and preliminary characterization. Biochem J 250:743–751
MacKintosh RW, Fewson CA (1988b) Benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase II from Acinetobacter calcoaceticus. Substrate specificities and inhibition studies. Biochem J 255:653–661
Mahajan MC, Phale PS, Vaidyanathan CS (1994) Evidence for the involvement of multiple pathways in the biodegradation of 1- and 2-methylnaphthalene by Pseudomonas putida CSV86. Arch Microbiol 161:425–433
Phale PS, Basu A, Majhi PD, Deveryshetty J, Vamsee-Krishna C, Shrivastava R (2007) Metabolic diversity in bacterial degradation of aromatic compounds. OMICS 11:252–279
Reid MF, Fewson CA (1994) Molecular characterization of microbial alcohol dehydrogenases. Crit Rev Microbiol 20:13–56
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Shaw JP, Harayama S (1990) Purification and characterisation of TOL plasmid-encoded benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase of Pseudomonas putida. Eur J Biochem 191:705–714
Shaw JP, Schwager F, Harayama S (1992) Substrate-specificity of benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase encoded by TOL plasmid pWW0. Metabolic and mechanistic implications. Biochem J 283(Pt 3):789–794
Shaw JP, Rekik M, Schwager F, Harayama S (1993) Kinetic studies on benzyl alcohol dehydrogenase encoded by TOL plasmid pWWO. A member of the zinc-containing long chain alcohol dehydrogenase family. J Biol Chem 268:10842–10850
Suhara K, Takemori S, Katagiri M (1969) The purification and properties of benzylalcohol dehydrogenase from Pseudomonas sp. Arch Biochem Biophys 130:422–429
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tasaki Y, Yoshikawa H, Tamura H (2006) Isolation and characterization of an alcohol dehydrogenase gene from the octylphenol polyethoxylate degrader Pseudomonas putida S-5. Biosci Biotechnol Biochem 70:1855–1863
Vangnai AS, Arp DJ, Sayavedra-Soto LA (2002) Two distinct alcohol dehydrogenases participate in butane metabolism by Pseudomonas butanovora. J Bacteriol 184:1916–1924
Vasiliou V, Pappa A, Petersen DR (2000) Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem Biol Interact 129:1–19
Vonck J, Arfman N, De Vries GE, Van BJ, Van Bruggen EF, Dijkhuizen L (1991) Electron microscopic analysis and biochemical characterization of a novel methanol dehydrogenase from the thermotolerant Bacillus sp. C1. J Biol Chem 266:3949–3954
Acknowledgments
PP and RS would like to thank Sophisticated Analytical Instrument Facility (SAIF), IIT-B, for ICP–AES analysis. Senior research fellowship to RS from IIT-B is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jan Roelof van der Meer.
R. Shrivastava and A. Basu contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shrivastava, R., Basu, A. & Phale, P.S. Purification and characterization of benzyl alcohol- and benzaldehyde- dehydrogenase from Pseudomonas putida CSV86. Arch Microbiol 193, 553–563 (2011). https://doi.org/10.1007/s00203-011-0697-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-011-0697-6