Abstract
Gluconacetobacter diazotrophicus, an endophyte isolated from sugarcane, is a strict aerobe that fixates N2. This process is catalyzed by nitrogenase and requires copious amounts of ATP. Nitrogenase activity is extremely sensitive to inhibition by oxygen and reactive oxygen species (ROS). However, the elevated oxidative metabolic rates required to sustain biological nitrogen fixation (BNF) may favor an increased production of ROS. Here, we explored this paradox and observed that ROS levels are, in fact, decreased in nitrogen-fixing cells due to the up-regulation of transcript levels of six ROS-detoxifying genes. A cluster analyses based on common expression patterns revealed the existence of a stable cluster with 99.8% similarity made up of the genes encoding the α-subunit of nitrogenase Mo–Fe protein (nifD), superoxide dismutase (sodA) and catalase type E (katE). Finally, nitrogenase activity was inhibited in a dose-dependent manner by paraquat, a redox cycler that increases cellular ROS levels. Our data revealed that ROS can strongly inhibit nitrogenase activity, and G. diazotrophicus alters its redox metabolism during BNF by increasing antioxidant transcript levels resulting in a lower ROS generation. We suggest that careful controlled ROS production during this critical phase is an adaptive mechanism to allow nitrogen fixation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane production in many countries depends on the traditional practice of adding nitrogen (NH3) fertilizer for optimum growth and yield. In regions of the world where nitrogen input is very low, the diazotrophic bacterium Gluconacetobacter diazotrophicus has often been isolated from surface-sterilized roots, stems and leaves (Baldani et al. 1997; Cavalcante and Dobereiner 1988). Studies trying to understand the importance of G. diazotrophicus in plants grown under these conditions showed that in a N-deficient environment, sugarcane plants inoculated with the strain PAl5 grew better and had a higher total N content than plants inoculated with the mutant nif- or uninoculated plants (Sevilla et al. 2001). These results indicate that the transfer of fixed N from G. diazotrophicus to sugarcane might be a significant mechanism to support plant growth.
BNF is the process by which N2 is converted into NH3 and is considered an energy-demanding process because it consumes 16 mol of ATP per mol of N2 (Kim and Rees 1994). This energy requirement can be met by aerobic respiration (Flores-Encarnacion et al. 1999). In this process, energy-rich substrates (nutrients) are oxidized, donating its electrons to a series of membrane proteins, known as the electron transport chain (ETC.), which will conserve the energy of the electron flow in the form of a membrane potential that will be the driving force to allow ATP synthesis. During respiration, molecular oxygen is the final electron acceptor, receiving 4 electrons, being reduced to water. However, some electrons may escape from the ETC., giving rise to partially reduced oxygen intermediates, commonly called reactive oxygen species (ROS) (Kowaltowski et al. 2009). Despite the need of high-energy supplies to support BNF, which is only achieved by respiration, nitrogenase is notoriously sensitive to oxygen and ROS (Robson and Postgate 1980).
So far, studies concerning the effects of oxygen on G. diazotrophicus have mainly dealt with the nature of its respiratory system. The most accepted hypothesis, called “respiratory protection”, postulates that the increase in O2 consumption during BNF would allow sufficient ATP production and at the same time lower intracellular oxygen levels, preserving nitrogenase activity (Dalton and Postgate 1968; Flores-Encarnación et al. 1999; Pan and Vessey 2001; Ureta and Nordlund 2002). Less data have been acquired concerning the influence of increased respiration on the production and detoxification of reactive oxygen species (ROS), a natural by-product of aerobic metabolism and its effects on BNF. The generation of partially reduced forms of oxygen (i.e. ROS), such as superoxide radical (O ·−2 ) and hydrogen peroxide (H2O2), may be part of the O2 toxicity problem in nitrogen-fixing organisms (Dalton 1995). Considering the lack of information about free radical biology during BNF and the importance of this process for sustainable agriculture, the aim of our work is to investigate the influence of ROS production and detoxification in G. diazotrophicus. Our data revealed that, besides the respiratory protection mechanism, G. diazotrophicus alters its redox metabolism during BNF by increasing antioxidant transcript levels resulting in a decreased ROS generation that is critical to sustain nitrogenase activity and allow BNF. As we show that ROS can strongly inhibit the BNF process, we suggest that careful control of ROS production during this critical phase is an adaptive mechanism to allow nitrogen fixation.
Materials and methods
Cultivation conditions
Gluconacetobacter diazotrophicus strain PAL5 (BR11281) was obtained from the Embrapa Agrobiologia Culture Collection and was grown in LGI-P medium on a shaker with gentle agitation (100 rpm) for 48 h at 30°C. After three successive rounds of cultivation, cells were harvested by centrifugation at 12,000g for 2 min and resuspended in LGI-P medium (0.2 g/l K2HPO4, 0.6 g/l KH2PO4, 0.2 g/l MgSO4 · 7H2O, 0.02 g/l CaCl2 · 2H2O, 0.002 g/l Na2MoO4 · 2H2O, 0.01 g/l FeCl3 · 6H2O, 100 g/l sacarose) with 1.0 mM of (NH4)2SO4 to yield an A600 of 0.6. Aliquots (1 ml) of cell suspensions were transferred into 250 ml flasks containing 50 ml of the fresh LGI-P medium (supplemented with 1.0 mM (nitrogen-fixing condition) or 20 mM of (NH4)2SO4 (non-nitrogen-fixing condition) then incubated at 30°C with agitation (100 rpm).
The resistance of G. diazotrophicus cells to oxidative stress was investigated by the addition of Paraquat (PQ), a superoxide generator (Hassett et al. 1987) to a final concentration of 0.5 or 5 mM in fixing and non-nitrogen-fixing cultures after 24 h cultivation.
Determination of ROS production in G. diazotrophicus
Free-radical production was assessed by fluorescence microscopy of cells from liquid cultures grown in LGI-P medium under fixing and non-nitrogen-fixing conditions. G. diazotrophicus cultures were threefold concentrated and incubated for 10 min in the presence of the redox-sensitive dye CM-H2DCFDA (5 μM) (Invitrogen—Molecular probes), followed by a washing step with fresh media and direct visualization (without fixative agents) of the samples. Images were acquired on a Zeiss Axioskop microscope with an Axiocam MRC5 using a Zeiss-09 filter set (excitation—BP 450–490; beam splitter—FT 510; emission—LP 515). Magnification was set at 630×.
Catalase activity
G. diazotrophicus was assayed for catalase activity according to (Aebi 1984). Briefly, crude extracts were incubated in Tris-buffer pH 7.0 in the presence of 9 mM hydrogen peroxide (H2O2) and absorbance was monitored at 240 nm for 1 min. The amount of protein was determined according to Bradford (1976).
Quantitative real-time polymerase chain reaction (qPCR)
The expression of selected genes was determined by real-time qPCR with SYBR Green Supermix (Applied Biosystems) using iCycler thermal cycler (Applied Biosystems). RNA was isolated with Trizol according to the manufacturer’s protocol. Samples were treated with DNase I (Invitrogen). Total RNA (2.5 μg) was used for cDNA synthesis with the Superscript First-strand synthesis kit (Invitrogen). cDNA was purified using MinElute PCR purification kit (Qiagen) and used as qPCR templates. Each PCR reaction contained 12.5 μl of 2× SYBR Green Supermix (Applied Biosystems), 1 μM primers and appropriate templates in a 25-μl reaction. PCR reactions were heated to 95°C for 3 min and then for 40 cycles with steps of 95°C for 30 s, 60°C for 15 s, 60°C for 45 s. The generation of specific PCR products was confirmed by the melting curve analysis and gel electrophoresis. The 2−ΔΔCt method (Livak and Schmittgen 2001) was employed for relative quantification and 23S rDNA was the housekeeping gene control. The results were based on the average of triplicate experiments. Primer sequences are available upon request.
Cluster analysis
We used qPCR to measure mRNA levels of cells grown under fixing or non-fixing conditions at 18, 24, 36, 48 and 72 h. The expressions of sodA, katE, kat, katC, gorA, gorB and nifD were measured at the same time points and the cluster analysis was also carried out at these times. Genes were grouped on the basis of their common expression patterns across the time points and different conditions.
Cluster analysis of gene expression was carried out with the MINITAB v.14 package, using Euclidian distances and the Ward method for linkage. The single, complete, average, centroid or median linkage methods displayed the same results in the test.
Nitrogenase activity
For the in vivo nitrogenase assay, 2 ml of G. diazotrophicus-saturated culture grown in LGI-P medium was used to inoculate 250-ml flasks containing 48 ml LGI-P supplemented with 1 mM of (NH4)2SO4. The cells were grown in a shaking water bath at 100 rpm for the indicated time at 30°C. The nitrogenase activity was determined in whole cells by the acetylene reduction method as described (Reis and Dobereiner 1998). Ethylene produced was analyzed by gas chromatography in a Perkin Elmer gas chromatograph equipped with a flame ionization detector and Porapak N column. The protein content was determined by the Bradford method (Bradford 1976).
Results
ROS levels are decreased during nitrogen fixation
To test our hypothesis that redox metabolism is altered during the BNF process, we evaluated the presence of oxygen radicals in G. diazotrophicus. Cells were stimulated to fix nitrogen and compared to a non-nitrogen-fixing condition. These two groups were loaded in the presence of the redox-sensitive fluorescent dye and observed under the microscope. Figure 1 shows that nitrogen-fixing cells (FIX—in Fig. 1) present a decreased fluorescent signal (1B and 1D), an indicative of lower ROS levels, when compared to non-nitrogen-fixing cells (NFIX) (1F and 1H), both at 24 and 48 h incubation time.
ROS levels are decreased in nitrogen-fixing cells. G. diazotrophicus grown under fixing (FIX) and non-fixing (NFIX) conditions were stained with CM-H2DCFDA, sensitive to ROS. a, c, e and g represent bright field images. b, d, f, h are the fluorescent images, an indicative of the presence of ROS. All images were acquired with the same exposure time to allow comparison of signal intensities. Scale bar 10 μm
These results demonstrate that cellular ROS are reduced when G. diazotrophicus is fixing nitrogen, pointing to a possible inverse correlation between the presence of ROS and nitrogenase activity. Furthermore, it is evident the non-fixing cells inhabit an environment where ROS is part of the cellular milieu without exhibiting any noticeable adverse effect. This is consistent with the notion that ROS act as physiological molecules in a diverse set of biological situations (Forman et al. 2010; Terada 2006), as it seems to occur in non-fixing G. diazotrophicus. It is tempting to speculate why ROS levels have to be reduced during BNF and we hypothesized that the sensitivity of nitrogenase to inhibition by oxygen intermediates may be in the center of this question.
ROS detoxification is up-regulated during BNF process
In order to investigate the mechanisms involved in the decrease of ROS during BNF, we first measured catalase activity, an antioxidant enzyme involved in H2O2 removal. Figure 2a shows increased activity in nitrogen-fixing cultures at 48 and 72 h. Considering that catalase activity was increased up to sixfold in fixing cells and at the same time ROS levels were greatly reduced (Fig. 1), we hypothesized that other antioxidant pathways may also be active during BNF. Genes that could possibly be involved in ROS detoxification were identified in the recently published G. diazotrophicus genome (Bertalan et al. 2009). Our analysis revealed the presence of six genes whose products are directly involved in ROS removal (Table 1).
Expression and activity of ROS detoxification in G. diazotrophicus during nitrogen fixation. a Catalase activity of cells grown in fixing and non-fixing conditions. b mRNA expression of the genes involved in ROS-detoxification from fixing and non-fixing cells. The data are expressed as the relative expression of the respective mRNAs normalized to the housekeeping gene 23S. The data are expressed as the average of three replicates ± error bars
qPCR used to measure mRNA levels of the identified ROS-detoxification genes in PAL-5 strain grown in high (NFIX) or low-nitrogen (FIX) media showed a strong induction of sodA, katE, kat, katC and gorA genes in response to nitrogen limitation (Fig. 2b). Importantly, genes that are not related to ROS metabolism (GS—glutamine synthetase—and amidase) do not have their expression levels altered during nitrogen fixation (Fig. 2b). Our results demonstrated a specific response of cells to reduce ROS levels under BNF conditions. Consistent with data presented in Fig. 1, non-fixing cultures seems to live in the presence of ROS and this condition does not represent a challenge since all the antioxidant enzymes analyzed were present in basal levels compared to fixing cells.
The genes for catalase type E (katE) and superoxide dismutase A (sodA) are co-expressed with the α-subunit of nitrogenase Mo–Fe protein (nifD)
In order to identify the most relevant ROS-detoxification genes to BNF, we searched for distinct expression patterns (clusters) (D’Haeseleer 2005). Genes were grouped on the basis of their common expressions across time points and under nitrogen-fixing and non-nitrogen-fixing conditions (Fig. 3). Cluster analysis separated these genes in two major clusters: one formed by gorB alone and the other formed by gorA, kat, katC, nifD, sodA and katE, with similarities up to 98% inside this group. Notice that the genes present in this cluster are strongly induced as mentioned earlier (Fig. 2b). This last cluster can be divided into three minor clusters kat + gorA; katC; and katE + sodA + nifD. This cluster has 99.8% similarity and it is stable when we use different algorithms for clustering (single and average linkage, median linkage and ward linkage), suggesting that sodA and katE have a major protective role in the nitrogen fixation process. It is interesting to note that the genes grouped in this cluster have higher similarity to related enzymes from other non-symbiontic closely related subclass Alphaproteobacteria (Table 1), while the other ROS-detoxifying genes analyzed have higher similarity to related enzymes from phylogenetically distant symbiontic organisms. This could be an indication that nitrogen fixation is an ancient process in G. diazotrophicus and was probably acquired before the adaptation to the endophytic lifestyle (Bertalan et al. 2009).
Cluster analysis of redox genes. Notice that there are two major clusters: one formed by gorB alone and the other formed by gorA, kat, katC, nifD, sodA, and katE, with similarities up to 98% inside this group. This last cluster can be divided in three minor clusters kat + gorA, katC and katE + sodA + nifD
The presence of ROS strongly inhibits nitrogen fixation
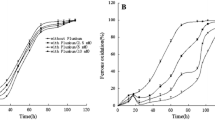
To further explore differences concerning ROS levels between nitrogen-fixing and non-fixing bacteria, the resistance of G. diazotrophicus to oxidative stress was investigated by the addition of Paraquat (PQ), a redox cycler that increases cellular ROS generation, into the cultures. The growth of nitrogen-fixing cells (Fig. 4a) was severely inhibited by the addition of PQ, indicating that the antioxidant capacity of fixing cells (Fig. 2) is overwhelmed by the pro-oxidant effects of this molecule. On the other hand, non-nitrogen-fixing cells were resistant to both 0.5 and 5 mM of PQ, as they maintained high growth rates and achieved high cell densities (Fig. 4a). General appearance of cells was similar for all PQ concentrations (data not shown). This data is in accordance with previous results (Figs. 1, 2) and demonstrate the resistance of non-fixing cells to ROS also reveals that fixing cells up-regulate antioxidant pathways (Fig. 2) to lower ROS levels (Fig. 1), a condition that is essential for its survival (Fig. 4a).
ROS effect on cell growth and BNF activity. a Effect of different concentrations of PQ on the growth of fixing (black) and non-fixing (gray) cells. After an initial growth period of 24 h, PQ was added at a concentration of 0.5 mM or 5 mM (dashed and dotted lines, respectively). b Acetylene reduction activity (ARA) of G. diazotrophicus after 72 h cultivation. The activity was measured after 48 h in the presence of 0.5 and 5 mM of PQ
To investigate whether BNF suppression was the basis for inhibition of cell growth in the presence of PQ, nitrogenase activity of cultures grown in the presence of PQ was tested by the acetylene reduction essay. Cells grown without PQ were used as a control. PQ was added to the medium after 24 h of growth and the nitrogenase activity was measured after a further 48 h (72 h of cultivation). Nitrogenase activity decreased 64% when 0.5 mM of PQ was added to the medium. When the concentration was changed from 0.5 to 5 mM, nitrogenase activity decreased to zero showing that the presence of ROS can strongly inhibit BNF in G. diazotrophicus (Fig. 4b). Therefore, fixing cells up-regulate antioxidant pathways in order to lower ROS levels and preserve nitrogenase activity, placing the regulation of ROS levels as a critical step to allow biological nitrogen fixation.
Discussion
Here, we demonstrate that antioxidant pathways are up-regulated during BNF to prevent ROS-induced nitrogenase inhibition in G. diazotriphicus. Organisms that fix nitrogen usually adopt different strategies to protect nitrogenase from contact with O2. The most studied is the Rhizobia strategy that infects the plant and forms nodules that create an anaerobic environment that favors BNF, while the plant supplies the energy requirement for the N-fixing bacteria (Fischer 1994). Differently from Rhizobia, G. diazotriphicus colonizes the intercellular spaces of the plant (apoplastic) and doesn’t form nodules and is therefore always exposed to higher levels of O2 (Dong et al. 1994). In fact, optimal nitrogen fixation by G. diazotrophicus demands high aerobic conditions both in solid (Pan and Vessey 2001) and liquid cultures (Flores-Encarnacion et al. 1999). However, when oxygen is measured in the liquid phase during BNF, the maximum nitrogenase activity of G. diazotrophicus is observed at a pO2 of 0.2 kPa (Reis and Dobereiner 1998). This is an indication that in order to keep the low levels of O2 dissolved in the medium G. diazotrophicus adopts the so-called “respiratory protection” strategy. Accordingly, G. diazotrophicus changes its electron transport chain composition during BNF. In this context, well-aerated cultures express cytochrome a1 and cytochrome bb as the main terminal oxidase. During repression of diazotrophic activity, cytochrome a1 diminishes dramatically concomitantly with the appearance of cytochrome bd (Flores-Encarnacion et al. 1999). Oxidase activities are also much higher in membrane preparations obtained from cultures under BNF conditions than in those from cultures under non-nitrogen-fixing conditions and the respiratory rate was among the highest ever reported for aerobic bacteria (Flores-Encarnacion et al. 1999; Gonzalez et al. 2006).
However, an aspect that has been overlooked is the impact of the respiratory protection on ROS generation. Higher respiratory rates may lead to increased production of oxygen radicals (Boveris and Chance 1973), a condition that would favor redox imbalance and consequently oxidative stress. Here, we tried to explore this paradox and incorporate our data into the respiratory protection hypothesis. We propose that in parallel to the increase in aerobic respiration that occurs during BNF, G. diazotrophicus also up-regulate antioxidant pathways to protect nitrogenase from ROS inhibition.
The importance of redox homeostasis to symbiotic BNF process has been indicated by previous studies with Sinorhizobium meliloti strains. Mutants affected in the antioxidant defense did not reach the differentiation stage of nitrogen-fixing bacteroids (Santos et al. 2000; Jamet et al. 2003; Harrison et al. 2005). Similarly, a peroxiredoxin (prxS)/bifunctional catalase–peroxidase (katG) Rhizobium etli double mutant has a significantly reduced symbiotic nitrogen fixation capacity (Dombrecht et al. 2005; for a review see Chang et al. 2009). Still, these effects could be both due to the presence of ROS or due to the incapacity of the bacteria to promote a normal nodule formation and therefore the environment that is necessary to allow nitrogen fixation. Our data show that ROS can directly inhibit nitrogenase activity, and G. diazotrophicus alters its redox metabolism during BNF to cope with that.
Concerning our gene analysis, most of the ORFs shared by G. diazotrophicus and other closely associated Alphaproteobacteria are related to energy metabolism and are generally part of the core genome of G. diazotrophicus (Bertalan et al. 2009). One interesting aspect of catalase gene analysis (GDI0467 and GDI2359) is that although the most similar sequences are from phylogenetically distant organisms, they were all isolated from plant leaves and have the ability to promote the growth of various plant seedlings (Abanda-Nkpwatt et al. 2006; Sessitsch et al. 2005). This is an indicative that these genes were acquired later, possible by lateral gene transfer. This could be especially important for bacterial adaptation to the endophytic lifestyle and may confer advantages to G. diazotrophicus in comparison with other microbes that colonize the same niche.
This study can be regarded as the first step toward understanding of ROS metabolism in Acetobacteraceae. It is also the first characterization of ROS production and modification of the redox state during BNF process. Our main finding is that during BNF G. diazotrophicus activate antioxidant pathways that diminish the intracellular levels of ROS protecting nitrogenase from ROS toxicity. These results complement the respiratory protection hypothesis previously described and allow a better comprehension of nitrogenase activity in aerobic environments.
References
Abanda-Nkpwatt D, Musch M, Tschiersch J, Boettner M, Schwab W (2006) Molecular interaction between Methylobacterium extorquens and seedlings: growth promotion, methanol consumption, and localization of the methanol emission site. J Exp Bot 57:4025–4032
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Baldani JI, Caruso L, Baldani VLD, Goi SR, Dobereiner J (1997) Recent advances in BNF with non-legume plants. Soil Biol Biochem 29:911–922
Bertalan M, Albano R, de Padua V, Rouws L, Rojas C, Hemerly A, Teixeira K, Schwab S, Araujo J, Oliveira A et al (2009) Complete genome sequence of the sugarcane nitrogen-fixing endophyte Gluconacetobacter diazotrophicus Pal5. BMC Genomics 10:450
Boveris A, Chance B (1973) The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134:707–716
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cavalcante VA, Dobereiner J (1988) A new acid-tolerant nitrogen-fixing bacterium associated with sugarcane. Plant Soil 108:23–31
Chang C, Damiani I, Puppo A, Frendo P (2009) Redox changes during the legume–Rhizobium symbiosis. Mol Plant 2:370–377
D’Haeseleer P (2005) How does gene expression clustering work? Nat Biotechnol 23:1499–1501
Dalton DA (1995) Antioxidant defenses of plants and fungi. Chapman & Hall, New York
Dalton H, Postgate JR (1968) Effect of oxygen on growth of Azotobacter chroococcum in batch and continuous cultures. J Gen Microbiol 54:463–473
Dombrecht B, Heusdens C, Beullens S, Verreth C, Mulkers E, Proost P, Vanderleyden J, Michiels J (2005) Defence of Rhizobium etli bacteroids against oxidative stress involves a complexly regulated atypical 2-Cys peroxiredoxin. Mol Microbiol 55:1207–1221
Dong Z, Canny MJ, McCully ME, Roboredo MR, Cabadilla CF, Ortega E, Rodes R (1994) A nitrogen-fixing endophyte of sugarcane stems (a new role for the apoplast). Plant Physiol 105:1139–1147
Fischer HM (1994) Genetic regulation of nitrogen fixation in rhizobia. Microbiol Rev 58:352–386
Flores-Encarnacion M, Contreras-Zentella M, Soto-Urzua L, Aguilar GR, Baca BE, Escamilla JE (1999) The respiratory system and diazotrophic activity of Acetobacter diazotrophicus PAL5. J Bacteriol 181:6987–6995
Forman HJ, Maiorino M, Ursini F (2010) Signaling functions of reactive oxygen species. Biochemistry 49:835–842
Gonzalez B, Martinez S, Chavez JL, Lee S, Castro NA, Dominguez MA, Gomez S, Contreras ML, Kennedy C, Escamilla JE (2006) Respiratory system of Gluconacetobacter diazotrophicus Pal5. Evidence for a cyanide-sensitive cytochrome bb and cyanide-resistant cytochrome ba quinol oxidases. Biochim Biophys Acta 1757:1614–1622
Harrison J, Jamet A, Muglia CI, Van de Sype G, Aguilar OM, Puppo A, Frendo P (2005) Glutathione plays a fundamental role in growth and symbiotic capacity of Sinorhizobium meliloti. J Bacteriol 187:168–174
Hassett DJ, Britigan BE, Svendsen T, Rosen GM, Cohen MS (1987) Bacteria form intracellular free radicals in response to paraquat and streptonigrin. Demonstration of the potency of hydroxyl radical. J Biol Chem 262:13404–13408
Jamet A, Sigaud S, Van de Sype G, Puppo A, Herouart D (2003) Expression of the bacterial catalase genes during Sinorhizobium meliloti–Medicago sativa symbiosis and their crucial role during the infection process. Mol Plant Microbe Interact 16:217–225
Kim J, Rees DC (1994) Nitrogenase and biological nitrogen fixation. Biochemistry 33:389–397
Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE (2009) Mitochondria and reactive oxygen species. Free Radic Biol Med 47:333–343
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Pan B, Vessey JK (2001) Response of the endophytic diazotroph Gluconacetobacter diazotrophicus on solid media to changes in atmospheric partial O2 pressure. Appl Environ Microbiol 67:4694–4700
Reis VM, Dobereiner J (1998) Effect of high sugar concentration on nitrogenase activity of Acetobacter diazotrophicus. Arch Microbiol 171:13–18
Robson RL, Postgate JR (1980) Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol 34:183–207
Santos R, Hérouart D, Puppo A, Touati D (2000) Critical protective role of bacterial superoxide dismutase in Rhizobium–legume symbiosis. Mol Microbiol 38:750–759
Sessitsch A, Coenye T, Sturz AV, Vandamme P, Barka EA, Salles JF, Van Elsas JD, Faure D, Reiter B, Glick BR et al (2005) Burkholderia phytofirmans sp. nov., a novel plant-associated bacterium with plant-beneficial properties. Int J Syst Evol Microbiol 55:1187–1192
Sevilla M, Burris RH, Gunapala N, Kennedy C (2001) Comparison of benefit to sugarcane plant growth and 15N2 incorporation following inoculation of sterile plants with Acetobacter diazotrophicus wild-type and Nif-mutants strains. Mol Plant Microbe Interact 14:358–366
Terada LS (2006) Specificity in reactive oxidant signaling: think globally, act locally. J Cell Biol 174:615–623
Ureta A, Nordlund S (2002) Evidence for conformational protection of nitrogenase against oxygen in Gluconacetobacter diazotrophicus by a putative FeSII protein. J Bacteriol 184:5805–5809
Acknowledgments
This work was supported by CNPq, FINEP, FAPERJ and INCT-FBN.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Stuart Ferguson.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Alquéres, S.M.C., Oliveira, J.H.M., Nogueira, E.M. et al. Antioxidant pathways are up-regulated during biological nitrogen fixation to prevent ROS-induced nitrogenase inhibition in Gluconacetobacter diazotrophicus . Arch Microbiol 192, 835–841 (2010). https://doi.org/10.1007/s00203-010-0609-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-010-0609-1