Abstract
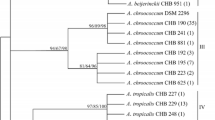
In order to promote the use of Azotobacter inoculants for cotton crop, a complete characterization of soil isolates of Azotobacter, isolated and screened on the basis of physiological properties, from four different cotton–wheat cropping regions of India was carried out, and their genetic diversity determined by RFLP (restriction fragment length polymorphism) analysis of the functional gene nifH. Genetic analysis of these isolates depicted a similarity coefficient of ≥80% among them, suggesting that though the isolates were obtained from different cotton soils of India, still they have large commonality in the nifH gene and constituted a homogeneous nifH population.


Similar content being viewed by others
References
Bashan Y, Puente ME, Rodriguez-Mendoza MN, Toledo G, Holguin G, Ferrera-Cerrato R, Pedrin S (1995) Survival of Azospirillum brasilense in the bulk soil and rhizosphere of 23 soil types. Appl Environ Microbiol 61:1930–1945
Chaney AL, Marbach EP (1962) Modified reagents for determination of urea and ammonia. Clin Chem 8:130–132
Clerc A, Manceau C, Nesme X (1998) Comparison of randomly amplified polymorphic DNA with amplified fragment length polymorphism to assess genetic diversity and genetic relatedness within genospecies III. Appl Environ Microbiol 64:1180–1187
Dalmastri C, Chiarini L, Cantale C, Bevivino A, Tabacchioni S (1999) Soil type and maize cultivar affect the genetic diversity of maize root-associated Burkholderia cepacia populations. Microb Ecol 38:273–284
Dart PJ, Subba Rao RV (1981) Nitrogen fixation associated with sorghum and millet. In: Vose PB, Rusehel AP (eds) Associative nitrogen fixation. CRC Press, Inc., Palm Beach, pp 169–177
Dobbelaere S, Vanderleyden J, Okon Y (2003) Plant growth promoting effects of diazotrophs in the rhizosphere. Critical Rev Plant Sci 22:107–149
Döbereiner J, Reis VM, Lazarini AC (1988) New N2-fixing bacteria in association with cereals and sugarcane. Gustav Fischer Verlag, Stuttgart, pp 717–722
Edi-Premono M, Moawad AM, Vlek PLG (1996) Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones J Crop Sci 11:13–23
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the salkowski reagent for indole compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796
Hardy RWF, Holstein RD, Jackson EK, Burns RC (1968) The acetylene-ethylene assay for nitrogen fixation: laboratory and field evaluation. Plant Physiol 43:1185–1207
Hartman A, Singh M, Klingmuler W (1983) Isolation and characterization of Azospirillum mutants excreting high amounts of indole acetic acid. Can J Microbiol 29:916–923
Herbert D, Phips PJ, Strange RE (1971) Chemical analysis of microbial cells. In: Norris JR, Ribbons DW (eds) Methods in microbiology. Academic Press, New York, pp 209–344
Jensen V (1951) Notes on the biology of Azotobacter. Proc Soc Appl Bacteriol 74:89–93
Khullar S, Chahal VPS (1976) Distribution of Azotobacter in the Punjab soils. J Res Punjab Agric Univ 13:398–403
Kumar V, Narula N (1999) Solubilization of inorganic phosphates and growth emergence of wheat as affected by Azotobacter chroococcum. Biol Fertil Soils 28:301–305
Lakshminaryana K (1993) Influence of Azotobacter on nitrogen nutrition of plants and crop productivity. Proc Indian Natl Sci Acad B59:303–308
Macnaughton SJ, Stephen JT, Venosa AD, Davis GA, Chang YJ, White DC (1999) Microbial population changes during bioremediation of an experimental oil spill. Appl Environ Microbiol 65:3566–3574
Mrkovacki N, Kovacav LN, Mezei S (2001) Application of microbiological preparation in sugarbeet production. A Period Sci Res Field Vegetable Crops 35:67–73
Narula N, Lakshminarayana K, Tauro P (1981) Ammonia excretion by Azotobacter chroococcum. Biotech Bioengg 23:467–470
Narula N, Kumar V, Singh B, Bhatia R, Lakshminaryana K (2005a) Impact of biofertilizers on grain yield in spring wheat under varying fertility conditions and wheat-cotton rotation. Arch Agron Soil Sci 51:79–89
Narula N, Saharan BS, Kumar V, Bhatia R, Bishnoi LK, Lather BPS, Lakshminarayana K (2005b) Impact of the use of biofertilizers on cotton (Gossypium hirsutum) crop under irrigated agro-ecosystem. Arch Agron Soil Sci 51:69–77
Nesme X, Vanecchoutte M, Orso S, Hoste B, Swings J (1995) Diversity and genetic relatedness with genera Xanthomonas and Stenotrophomonas using restriction endonuclease site differences of PCR amplified 16S rRNA gene. Syst Appl Microbiol 18:127–135
Paul EA, Clark FE (1996) Soil microbiology and biochemistry, 2nd edn. Academic Press, San Diego, California
Rajakumar K, Lakshmanan M (1995) Influence of temperature on the survival and nitrogen fixing ability of Azotobacter chroococcum. Indian J Microbiol 35:25–30
Sindhu SS, Lakshminarayana K (1986) Distribution of Azotobacter in the Haryana soils and effect of bacteriostasis on Azotobacter survival. Environ Ecol 4:536–540
Staley JT, Boone DR, Brenner DJ, Castenholz RW, Garrity GM, Goodfellow M, Krieg NR, Rainey FA, Schleifer K (2001) Bergey’s manual of systematic bacteriology, 2nd edn. Springer, New York
Ueda T, Suga Y, Yahiro N, Matsuguchi T (1995) Remarkable N2-fixing bacterial diversity detected in rice roots by molecular evolutionary analysis of nifH gene sequences. J Bacteriol 177:1414–1417
Welbaum GE, Sturz AV, Dong Z, Nowak J (2004) Managing soil microorganisms to improve productivity of agro-ecosystems. Critical Rev Plant Sci 23(2):75–193
Yohalem DS, Lorbeer JW (1994) Intraspecific metabolic diversity among strains of Burkholderia cepacia isolated from decayed onions, soils and the clinical environment. Antonie Van Leeuwenhoek 65:111–131
Zehr JP, Capone DG (1996) Problems and promises of assaying the genetic potential for nitrogen fixation in the marine environment. Microb Ecol 32:263–281
Acknowledgments
The authors would like to acknowledge the help received from National Agricultural Technology Project (NATP) and German Academic Exchange Service (DAAD) during the course of this investigation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Rights and permissions
About this article
Cite this article
Bhatia, R., Ruppel, S. & Narula, N. NifH-based studies on azotobacterial diversity in cotton soils of India. Arch Microbiol 191, 807–813 (2009). https://doi.org/10.1007/s00203-009-0508-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-009-0508-5