Abstract
To determine and appraise the certainty of fracture liaison service (FLS) in reducing the risk of secondary fragility fractures in older adults aged ≥ 50 years and to examine the nature of the FLS and the roles of various disciplines involved in the delivery of the FLS. Medline, EMBASE, PubMed, CINAHL, SCOPUS, and The Cochrane Library were searched from January 1st, 2010, to May 31st, 2022. Two reviewers independently extracted data. The risk of bias was evaluated using the Newcastle–Ottawa Scale for cohort studies and the PEDro scale for randomized trials, while the GRADE approach established the certainty of the evidence. Thirty-seven studies were identified of which 34 (91.9%) were rated as having a low risk of bias and 22 (59.5%) were meta-analyzed. Clinically important low certainty evidence at 1 year (RR 0.26, CI 0.13 to 0.52, 6 pooled studies) and moderate certainty evidence at ≥ 2 years (RR 0.68, CI 0.55 to 0.83, 13 pooled studies) indicate that the risk of secondary fragility fracture was lower in the FLS intervention compared to the non-FLS intervention. Sensitivity analyses with no observed heterogeneity confirmed these findings. This review found clinically important moderate certainty evidence showing that the risk of secondary fragility fracture was lower in the FLS intervention at ≥ 2 years. More high-quality studies in this field could improve the certainty of the evidence. Review registration: PROSPERO―CRD42021266408.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The World Health Organization (WHO) reports that osteoporosis contributes to nearly 9 million fractures annually [1], burdening healthcare settings, resulting in major economic consequences for society [2], and potentially negatively impacting the quality of life of persons who have sustained a fracture [3]. Between 2015 and 2016, there were 18,746 new diagnoses of hip fractures and 93,321 hospitalizations of patients with a minimal trauma fragility fracture in Australia [4]. In the United Kingdom (UK), about half a million people sustain a fragility fracture annually [5], and it is estimated that there are 10 million people affected by osteoporosis annually in the United States [6]. It is expected that the number of older adults with fragility fractures will increase due to osteoporosis, a condition characterized by low bone mineral density (BMD) and deterioration of bone tissue [5]. Osteoporosis is a leading cause of fragility fractures in older adults [5]. As bones age, they become less dense and lose strength, making them more prone to fracture with less force or trauma [7]. Fragility fractures occur due to osteoporotic changes such as low BMD, which can result from a variety of factors such as aging, underlying medical conditions, and certain medications [7]. Other medical conditions that can lead to low BMD and increase the risk of fragility fractures include hyperparathyroidism, hyperthyroidism, malabsorption disorders, and certain cancers [7]. A report prepared by Hernlund et al. (2013) in collaboration with the international osteoporosis foundation has reported that a majority of individuals diagnosed with one fracture related to osteoporosis are not identified or treated for osteoporosis [8].
Certain medications, such as long-term use of glucocorticoids, can also lead to low BMD and increase the risk of fragility fractures [9]. In addition to these medical factors, lifestyle factors such as a sedentary lifestyle, smoking, excessive alcohol consumption, and low intake of calcium, vitamin D, and protein supplements can also contribute to the development of osteoporosis and increase the risk of fragility fractures [9].
The economic burden of fragility fractures is expected to increase as the population ages, and the prevalence of osteoporosis and other risk factors for fractures increases [9]. Fragility fractures can lead to prolonged hospital stays, increased healthcare utilization, and decreased quality of life. Older adults who experience fragility fractures have an increased risk of mortality and disability. In addition to the direct costs of treating fragility fractures, there are also indirect costs such as lost productivity and caregiver burden. Reports from Asia–Pacific, Eurasia, Europe, Latin America, and the United States of America show that the burden of the economic costs incurred to treat fragility fractures is high [9].
In 2018, the Asian Federation of Osteoporosis Societies (AFOS) estimated that the 1.1 million cases of hip fractures in 2018 will more than double to 2.5 million cases by 2050. The projected cost is to increase from US $7.4 billion in 2018 to almost US $13 billion in 2050 [10]. In addition, the Working Group for the Audit on the Burden of Osteoporosis in the Eurasian Region also reported projected increases in hip fractures ranging from 60 to 360% in some countries, for example, 310% in the Kyrgyz Republic and 360% in Uzbekistan [11]. Likewise, the European Union including Switzerland and the United Kingdom has estimated that there will be 4.3 million new fragility fractures in 2019 with a staggering cost of treating the fractures at Euro 56.9 billion (US $60.8 billion) [2].
The magnitude of the burden of fragility fractures in 2018 in people aged between 50 and 89 in Argentina, Brazil, Columbia, and Mexico was estimated at 840,000 fractures with an associated cost of almost US $1.2 billion ranging from US $411 million in Mexico to US $94 million in Columbia in 1 year [12]. Furthermore, an analysis commissioned in 2019 by the National Osteoporosis Foundation (renamed “Bone Health and Osteoporosis Foundation”) reported that during a 2-to-3-year follow-up period among Medicare fee-for-service beneficiaries who sustained a second fragility fracture, cost Medicare was estimated at US $6.3 billion [13]. Although the cost of sustaining a secondary fragility fracture is high, a report prepared by Hernlund et al. (2013) in collaboration with the International Osteoporosis Foundation has reported that a majority of individuals diagnosed with one fracture related to osteoporosis are not identified or treated for osteoporosis in Europe, despite fracture liaison service (FLS) programs being present in those countries [8]. With the high burden of fragility fractures and the predicted increase in the number of people with osteoporosis, secondary fragility fractures must be prevented [9, 14]. The International Osteoporosis Foundation’s global flagship program “Capture the Fracture” has over 800 FLS programs spread to 52 countries [14]. These services, programs, or models are designed to reduce the risk of secondary fractures [14]. While FLS has been implemented, the SCOPE 2021 scorecard for osteoporosis in Europe shows that many European countries will experience an increase in osteoporotic fractures from 4.28 million in 2019 to 5.05 million in 2034. The countries with the highest increase in the annual number of fractures in 2034 are Germany with a projected 931,000 fractures and Italy with about 666,000 fractures [2]. These significant findings indicate that identifying effective treatment strategies to prevent secondary fragility fractures is warranted.
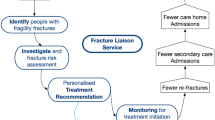
FLS models have been shown in various studies to be cost-effective in the prevention of secondary fragility fractures when used in a systematic approach with a fracture liaison coordinator [1, 5, 8]. They provide cost-effectiveness to the health service through fewer fractures and improved quality of life for patients [1, 5]. Evidence in various studies and best practice guidelines have shown that these services address the “osteoporosis treatment gap” by being an effective coordinated multidisciplinary liaison service that identifies, investigates, and treats fragility fractures [1, 5].
The FLS model originated in Scotland and is now utilized worldwide in the UK, Netherlands, Canada, USA, and Australia [1, 5]. Osuna et al [6]. provided a grouping system to determine the characteristics of FLS by categorizing them into four types of FLS. Type A is described as a service that identifies, initiates, and instigates treatments [6]. This type is the most intensive and comprehensive model being coordinated through a collaborative approach involving a lead champion (endocrinologist, orthopedic surgeon, or rheumatologist), a fracture liaison coordinator, physical and occupational therapists, and laboratory and radiological professionals [6]. In the type-A model, patients at risk are identified after the first fragility fracture and referred to the FLS [6]. The type-A FLS uses a coordinated and multi-disciplinary systematic approach to identify and evaluate patients who have a fragility fracture [6]. Evaluation usually consists of bone mineral density (BMD) using dual-energy x-ray absorptiometry (DXA), and blood tests to determine calcium and vitamin D levels are also undertaken [1, 8]. Treatment with bisphosphonates or a more recent drug, denosumab, is considered and initiated in patients based on a fracture risk assessment [1, 8]. Assessing fall risk and calcium and vitamin D supplementation needs is considered along with diet and lifestyle management interventions and education [1, 8]. This systematic collaborative approach is considered best practice [1, 5, 6]. The type-B FLS model is a service that identifies and investigates patients but then refers to the primary care service for treatment initiation and ongoing care [6]. The type-C model identifies patients at risk for further fragility fractures and informs the primary care physician and the patient [6]. Type-D identifies patients at risk of further fragility fractures and provides education and information to the patient without communicating their findings to the primary care physician or other members of the multi-disciplinary team [6].
Several systematic reviews have been conducted to determine the effectiveness of an FLS in reducing secondary fragility fractures [6, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. These reviews, however, largely focused on FLS coordinators and show that a variety of models for the FLS exist and describe the different approaches to how clients are managed and followed up. Although the previous reviews [16, 22, 30] collated data on secondary fragility fractures, these reviews were not prospectively registered, did not appraise the certainty of the evidence, and findings were not interpreted based on clinical significance. These outcomes indicate that a fresh systematic review that will appraise the certainty of the current evidence and interpret findings based on clinical relevance is needed to determine the best evidence synthesis on the effectiveness of an FLS in reducing secondary fragility fractures in older adults. Accordingly, the purpose of this systematic review was to determine and appraise the certainty of an FLS in reducing the risk of secondary fragility fractures in community-dwelling older adults aged greater or equal to 50 years and to examine the nature of the FLS and the roles of various disciplines involved in the delivery of the FLS.
Materials and methods
Study design
This systematic review was registered with the International Prospective Register for Systematic Reviews (PROSPERO―CRD42021266408) [31] before commencement. The Meta-analysis Of Observational Studies in Epidemiology (MOOSE) checklist was used to report the findings [32].
Data sources
Keywords and the search strategies were developed (eTable 1), and an electronic database search of titles and abstracts was conducted on the following databases: Medline, EMBASE, PubMed, CINAHL, SCOPUS, and The Cochrane Library from January 1st, 2010, to May 31st, 2022. Google scholar citation tracking and manual searches of the reference lists of the included articles were also performed, and duplications were removed using EndNote™ X9 [33]. All searches (both database and manual) were performed by one author (NL) who had a Bachelor of Science in Nursing with support from GD who had a PhD.
Eligibility criteria
Articles were eligible for inclusion (eTable 2) if they met the following criteria: (1) full reports of randomized or non-randomized studies that investigated the effects of FLS interventions compared to non-FLS interventions, (2) included community-dwelling older adults (≥ 50 years) with an index fragility fracture of any type and/or location, and (3) reported secondary fragility fracture or refracture rate as an outcome. The duration of outcome follow-up was categorized as short-term (less than 3 months after randomization), intermediate (from 3 months to less than 1 year), or long-term (1 year or more) [34]. A previous systematic review indicated that the median follow-up duration in FLS studies is 2 years with the most benefit being demonstrated in studies with 2 or more years of follow-up [22, 35]. For this reason, we divided the long-term follow-up duration into two; long-term follow-up at 1 year and long-term follow-up at 2 years or longer.
Study selection and data extraction
Study selection was performed by two independent reviewers (MD and GD). After the removal of duplicate records, full copies of potentially eligible papers were retrieved, screened, and extracted by the reviewers with disagreements resolved through consensus. During the review process, if the research team encountered differing viewpoints or interpretations of the data, and to ensure the objectivity and reliability of this review, these disagreements were addressed through consensus including (1) a review of the agreed-upon protocol, (2) noting of discrepancies or disagreements emerging during independent screening, (3) discussion of the differences in assessments and data extraction to provide an opportunity for reviewers to present their arguments and evidence supporting their interpretations, and (4) weighing the evidence, discussing the strengths and limitations of individual studies, and arriving at a shared interpretation. A mediator was not invoked as the researchers did not experience diverging viewpoints. In the case of missing data or uncertainties in the eligibility criteria or results, the corresponding authors were contacted directly.
Risk of bias assessment
Eligible studies were critically appraised for the risk of bias by two independent reviewers using the Newcastle–Ottawa Scale (NOS) for cohort studies [36, 37] and the Physiotherapy Evidence Database (PEDro) Scale for randomized trials [38] (Table 1), with disagreements resolved through consensus as reported above. This was a deviation from the original protocol which specified the use of the Jonna Brig’s Institute (JBI) Scale for quality appraisal. We found that NOS [36, 37] and PEDro scale [38] (eMethod) are more valid and reliable and are easier to rate by the reviewers than the JBI scale which is rather complex and has no standardized scoring criteria.
Certainty of the evidence
The Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) approach was used to rate the certainty of the evidence [34]. The certainty of the evidence for each comparison was downgraded by one level in the presence of study limitations [39], inconsistency of results [40], indirectness [41], imprecision of results [42], and publication bias [43] (eMethod).
Data synthesis
We systematically grouped studies for data synthesis (eTable 3). Following the groupings, two or more studies that reported sufficient data (sample size and frequency/percentage of subsequent fractures) and homogeneity in design (pre-FLS vs post-FLS design), intervention (FLS), comparison (non-FLS intervention), outcome (secondary fragility fracture/refracture rate), and follow-up periods (short, intermediate, and long-term follow-ups) were pooled into a meta-analysis using the RevMan-5.4 software. To minimize heterogeneity, a random effect model (when I2 was ≥ 50%) or fixed-effect model (when I2 was ≤ 50%) was used during the meta-analyses, and sensitivity analyses were also performed [34, 43]. We also assessed heterogeneity using (1) visual inspection of the overlap of the confidence intervals for individual studies in the forest plot; (2) Chi [2] test, with a low p-value (< 0.10) providing evidence of heterogeneity; and (3) I2 statistic, 0 to 40%, might not be important; 30 to 60%, may represent moderate heterogeneity; 50 to 90%, may represent substantial heterogeneity; and 75 to 100%, considerable heterogeneity [34]. We have also considered the magnitude and direction of the effects. The treatment effects and 95% confidence intervals were calculated using relative risk (RR), and RR > 1.25 or < 0.75 was considered clinically important [42, 44].
Sensitivity analyses
Sensitivity analyses were also performed to determine the consistency of the meta-analysis if sufficient data were available [34]. First, outliers were planned to be removed when the confidence interval of an individual study did not overlap with the meta-analysis confidence interval [45]. Second, sensitivity analyses were performed using the leave-one-out approach in which studies with low (I2 ≤ 25%) to high heterogeneity (I2 ≥ 75%) were identified and removed from the analyses [34]. Third, meta-analyses were also planned to be performed by removing studies that were rated as having a high risk of bias (Table 1) [34].
Subgroup analyses
We performed subgroup analyses to determine the impact of removing non-guideline-based FLS interventions from the initial meta-analyses to establish if guideline-based FLS interventions could be used as sole treatments.
Results
Study identification
The initial database search resulted in 599 citations, of which 274 were appropriate for full-text review. After a full-text review, 237 studies were excluded for not meeting one or more eligibility criteria (eTable 4), resulting in a total of 37 studies [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82] eligible for inclusion in this review (Fig. 1).
PRISMA flow diagram. From Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097(for more information, visit www.prisma-statement.org)
Characteristics of the included studies
A summary of the characteristics of the included studies is presented in eTable 5. Thirty-seven studies [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82] conducted with 115,381 participants (mean age = 53.92; SD = 8.26) diagnosed with index fragility fractures were included in the review. The sample size of the studies included ranged from 81 to 10,873 (median = 393; interquartile range = 199 to 3160) participants. Of the included studies, 19 (51.4%) [46,47,48,49, 51,52,53,54,55,56, 59, 62, 66, 70, 73,74,75, 77, 82] were prospective cohorts, 15 (40.5%) [50, 57, 60, 63,64,65, 67,68,69, 71, 72, 78,79,80,81] were retrospective cohorts, two (5.4%) [61, 76] were randomized trials, and one (2.7%) [58] was a prospective and retrospective parallel cohort. All the included studies reported secondary fragility fractures and used pharmacological interventions in their FLS programs. Additionally, 35 (94.6%) studies [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63, 65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80, 82] followed-up participants for at least 1 year, one study [64] terminated follow-up at 6 months, and one study [81] did not report follow-up duration. Most of the studies included (23; 62.2% studies) [46,47,48,49,50,51, 55, 56, 60,61,62,63, 65, 66, 68, 69, 71, 72, 74, 76, 79,80,81] did not report how the follow-up was conducted. However, five studies (13.5%) [53, 54, 59, 67, 78] conducted follow-up via in-person visits, five studies (13.5%) [57, 58, 64, 75, 82] via phone calls, three studies (8.1%) [70, 73, 77] via in-person visits or phone calls, and one study (2.7%) [52] via phone calls or postal surveys.
Risk of bias results
The risk of bias for included studies is reported in Table 1. The mean NOS score was 7.3 out of 9, with a range of 4 to 9 while the mean PEDro score was 6.5 out of 10, with a range of 6 to 7. Overall, 34 (91.9%) studies [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67, 69,70,71,72,73,74,75,76,77, 79, 80, 82] were rated as having a low risk of bias based on achieving a NOS or PEDro score of 6 or more. Common sources of bias were failure to recruit non-exposed cohorts or controls (15; 40.5% studies) [57, 68,69,70,71,72,73,74,75, 77,78,79,80,81,82], failure to match cohorts (exposed) with controls (15; 40.5% studies) [57, 68,69,70,71,72,73,74,75, 77,78,79,80,81,82], failure to adjust for confounders in the analysis (17; 45.9% studies) [46, 47, 50, 57, 59, 63, 68, 70, 73,74,75, 77,78,79,80,81,82], failure to account for the enrolled participants (10; 27.0% studies) [48, 49, 51, 58, 63, 64, 68, 69, 71, 78], failure to blind treating practitioners (2; 5.4% studies) [61, 76], failure to blind outcome assessors (2; 5.4% studies) [61, 76], and failure to blind participants (2; 5.4% studies) [61, 76].
Characteristics of the FLS interventions
The included studies in this systematic review were published between 2011 and 2022, which indicated that the timing of the data collection would have also influenced the findings of this review. All the studies included incorporated pharmacological interventions in their FLS programs, with 25 (67.6%) studies [46,47,48,49,50,51,52,53,54,55,56, 58, 59, 63,64,65,66, 69, 71, 74,75,76,77, 80, 82] administering interventions according to international guidelines (eTable 5). Additionally, several guidelines were reported, and no more than two studies used the same guidelines, and in all cases, those studies were from the same authors; Thailand Osteoporotic Foundation Guidelines (Amphansap et al.) [46, 47], Swedish Treatment Guidelines (Axelsson et al.) [48, 49], European and IOF guidelines (González-Quevedo et al.) [53, 54], Dutch Osteoporosis Guidelines (Huntjens et al.) [55, 56], and Catch a Break Guideline (Majumdar et al.) [75, 76]. The most commonly prescribed anti-osteoporotic medications included bisphosphonates (23; 62.2% studies) [46,47,48,49, 52,53,54, 56, 59, 63, 65, 66, 70, 71, 73,74,75,76,77,78,79, 81, 82], denosumab (10; 27.0% studies) [46,47,48,49, 53, 59, 74, 77, 81, 82], teriparatide (10 studies) [46,47,48, 53, 59, 63, 73, 74, 77, 81], raloxifene (3; 8.1% studies) [59, 73, 81], strontium ranelate (3; 8.1% studies) [46, 66, 82], calcitonin nasal spray (1; 2.7% studies) [46], and hormonal replacement therapy (1; 2.7% studies) [73]. Vitamin D and calcium supplementations were also combined when appropriate (17; 45.9% of studies) [46, 47, 52,53,54, 56,57,58,59, 64,65,66,67, 74, 77, 78, 81]. However, bisphosphonates which were received by 9823 participants were in most cases the first-line treatment for patients with normal renal function, while denosumab (received by 544 participants) was prescribed for patients with renal impairment or as a second-line treatment [46,47,48,49, 53, 59, 74, 77, 81, 82]. Investigations for osteoporosis included DEXA (22; 59.5% studies) [48,49,50, 53,54,55,56,57, 59, 62, 63, 65, 67, 70, 71, 73, 74, 77,78,79, 81, 82] and blood testing to rule out secondary causes of osteoporosis (12; 32.4% studies) [53, 54, 57, 59, 64, 66, 67, 73, 74, 77, 81, 82].
Nineteen studies (51.4%) [48,49,50, 53,54,55,56, 58,59,60, 62, 63, 65, 66, 68, 75,76,77,78] captured those presenting to the emergency department, 12 studies (32.4%) [46, 47, 51, 52, 57, 70,71,72, 79,80,81] captured those admitted to hospital, three studies (8.1%) [61, 73, 74] captured those admitted to hospital and those presenting to emergency department, two studies (5.4%) [64, 82] captured those presenting to orthopedic clinic, and one study (2.7%) [67] captured those presenting to neurosurgery clinic. Thirteen studies (35.1%) [48,49,50, 52, 57, 59,60,61, 64, 66, 70, 74, 77] reported fractures of any location, 11 studies (29.7%) [46, 47, 51, 53, 54, 63, 71, 72, 78,79,80] reported hip fractures, three studies (8.1%) [65, 67, 81] reported vertebral fractures, three studies (8.1%) [55, 56, 62] reported non-vertebral fractures, four studies (10.8%) [58, 69, 73, 82] did not report fracture types, one study (2.7%) [68] reported fractures of the distal radius, one study (2.7%) [76] reported upper extremity fractures, and one study (2.7%) [75] reported non-hip fractures. Most of the reviewed studies (25, 67.6% studies) [46, 47, 50,51,52,53,54,55,56,57, 60, 62,63,64, 66, 67, 69, 70, 73, 74, 77,78,79,80,81] used type-A FLS programs, with seven (18.9%) [48, 49, 59, 65, 71, 72, 82], three (8.1%) [58, 61, 68], and two (5.4%) [75, 76] studies using types B, C, and D FLS programs, respectively.
FLS practitioners
Most of the included studies reported the practitioners involved in the FLS programs (33; 89.2% studies) [46,47,48,49,50,51,52, 55,56,57,58,59,60,61,62, 64,65,66,67,68, 70,71,72,73,74,75,76,77,78,79,80,81,82], with nurses (19; 57.6% studies) [46, 47, 55,56,57, 62, 64,65,66, 70, 72,73,74,75,76,77,78,79, 82], physicians (2; 6.1% studies) [48, 50], general practitioners (2; 6.1% studies) [60, 68], rheumatologists (2; 6.1% studies) [59, 81], or orthogeriatrician (1; 3.0% study) [72] being the most commonly reported FLS coordinators. These coordinators were supported in most cases by physicians (7; 36.8% studies) [46, 47, 64, 70, 75, 76, 79], orthopedic surgeons (5; 26.3% studies) [56, 57, 64, 66, 74], general practitioners (2; 10.5% studies) [55, 82], endocrinologists (1; 5.3% studies) [82], traumatologists (1; 5.3% studies) [74], bone-health specialists—physicians who specialized in the management of osteoporosis and other bone-mineral diseases (1; 5.3% studies) [65], rheumatologists (1; 5.3% studies) [66], and multidisciplinary team (2; 10.5% studies) [70, 77] (eTable 5). The primary functions of the FLS coordinators included using in-hospital assessments to determine the patients who are at high risk of osteoporosis or refracture, providing patient education (diets, exercise, bone health), scheduling regular follow-ups, and updating the database [46, 47, 55,56,57, 62, 64,65,66, 70, 72,73,74,75,76,77,78,79, 82]. The primary functions of the prescribing practitioners included the initiation of drug treatment for fracture risk reduction, explaining to the patient the importance of drug therapy, and liaising with other health professionals such as radiologists (for medical imaging), laboratory scientists (for blood tests), and physiotherapists (to improve balance and muscular strength and prevent falling) [46, 47, 55,56,57, 64,65,66, 70, 74,75,76, 79, 82].
Data synthesis results
Meta-analyses were performed for 22 (59.5%) studies [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] that provided sufficient intermediate-term and long-term follow-up data for FLS interventions compared to non-FLS interventions. All the meta-analyzed studies were rated as having a low risk of bias based on achieving a NOS or PEDro score of 6 or more (Table 1). Of the meta-analyzed studies, 17 (77.3%) administered FLS according to international guidelines [46,47,48,49,50,51,52,53,54,55,56, 58, 59, 63,64,65,66], 13 (59.1%) used DEXA for the diagnosis of osteoporosis [48, 49, 53,54,55,56,57, 59, 62, 63, 65, 67], 12 (54.5%) prescribed bisphosphonates as the first-line treatment [46,47,48,49, 52,53,54, 56, 59, 63, 65, 66], and eight (36.4%) had nurses as the FLS coordinators [46, 47, 55, 56, 64,65,66]. Additionally, 13 (59.1%) studies were prospective cohorts [46,47,48,49, 51,52,53,54,55,56, 59, 62, 66], seven (31.8%) were retrospective cohorts [50, 57, 60, 63,64,65, 67], one (4.5%) was a randomized trial [61], and one (4.5%) was a prospective and retrospective parallel cohort [58]. All forest plots and GRADE ratings are presented in Table 2 and descriptive summaries are provided below in relevant subsections.
-
1.
Intermediate-term (< 1 year but ≥ 3 months)
There was moderate certainty evidence (publication bias) from five pooled studies (6590 participants) [48, 53, 58, 61, 64] showing no significant difference (RR 0.98, CI 0.83 to 1.16) between FLS and non-FLS groups in the risk of secondary fragility fracture in the intermediate-term follow-up.
-
2.
Long-term (at 1 year)
There was clinically important low certainty evidence (inconsistency and publication bias) from 6 pooled studies (1520 participants) [46, 47, 58, 61, 65, 66] showing that the risk of secondary fragility fracture was 74% lower (RR 0.26, CI 0.13 to 0.52) in the FLS group compared to the non-FLS group at 1-year follow-up.
-
3.
Long-term (at 2 years and above)
There was clinically important moderate certainty evidence (inconsistency) from 13 pooled studies (33,811 participants) [49,50,51,52, 54,55,56, 59,60,61,62,63, 67] showing that the risk of secondary fragility fracture was 32% lower (RR 0.68, CI 0.55 to 0.83) in the FLS group compared to the non-FLS group at 2 years and above.
Sensitivity analysis results
All the meta-analyzed studies were rated as having a low risk of bias making sensitivity analyses by removing studies with a high risk of bias irrelevant. Additionally, none of the meta-analyses contained studies with a confidence interval that failed to overlap with the confidence interval of the pooled studies, so the outlier sensitivity analysis did not change any findings. However, sensitivity analyses were performed by removing studies with low to high heterogeneity from the initial meta-analyses. All forest plots and GRADE ratings are presented in Table 3, and descriptive summaries are provided below in relevant subsections.
-
1. Intermediate term (< 1 year but ≥ 3 months).
Sensitivity analysis (I2 = 0%) by removing one study [58] showed moderate certainty evidence (publication bias) from four pooled studies (6,343 participants) [48, 53, 61, 64] supporting the meta-analysis of no significant difference (RR 1.00, CI 0.84 to 1.18) between FLS and non-FLS groups in the risk of secondary fragility fracture among individuals aged 50 years or older.
-
2. Long-term (at 1 year)
Sensitivity analysis (I2 = 0%) by removing two studies [46, 47] showed clinically important moderate certainty evidence (publication bias) from four pooled studies (932 participants) [58, 61, 65, 66] supporting the meta-analysis of the superiority of FLS intervention over non-FLS intervention in reducing secondary fragility fracture by 62% (RR 0.38, CI 0.25 to 0.58) among individuals aged 50 years or older.
-
3. Long-term (at ≥ 2 years)
Sensitivity analysis (I2 = 0%) by removing four studies [49, 56, 63, 67] showed clinically important moderate certainty evidence (publication bias) from nine pooled studies (7984 participants) [50,51,52, 54, 55, 59,60,61,62] supporting the meta-analysis of the superiority of FLS intervention over non-FLS intervention in reducing secondary fragility fracture by 28% (RR 0.72, CI 0.62 to 0.83) among individuals aged 50 years or older.
Subgroup analysis results
Subgroup analyses (Fig. 2) by removing non-guideline-based FLS interventions from the initial meta-analyses revealed that guideline-based FLS interventions could stand alone because they did not alter the results of the initial meta-analyses at intermediate-term (RR 0.99, CI 0.83 to 1.17; I2 = 28%; p = 0.87; four pooled studies; Fig. 2a) [48, 53, 58, 64], 1 year (RR 0.23, CI 0.11 to 0.50; I2 = 72%; p = 0.0002; five pooled studies; Fig. 2b) [46, 47, 58, 65, 66], and ≥ 2 years (RR 0.63, CI 0.48 to 0.83; I2 = 82%; p = 0.00008; nine pooled studies; Fig. 2c) [49,50,51,52, 54,55,56, 59, 63].
Publication bias results
Funnel plots showed symmetrical distributions for both meta-analysis comparisons at the intermediate-term (< 1 year but ≥ 3 months) and long-term (2 years and above) follow-ups, except for long-term follow-up comparison at 1 year which showed asymmetry. However, the small number of pooled studies (less than 10) at the intermediate-term follow-up indicated that this comparison could potentially be biased and was therefore downgraded (Table 2). Additionally, the funnel plots for sensitivity analyses (eFigure 1) showed symmetrical distributions at all timelines; however, these were also downgraded due to the small number of pooled studies in those comparisons (Table 3).
Discussion
This systematic review was conducted to determine and appraise the certainty of an FLS in reducing the risk of secondary fragility fractures in community-dwelling older adults aged ≥ 50 years and to examine the nature of the FLS and the roles of various disciplines involved in the delivery of the FLS. This review identified 37 studies, of which 25 (67.6%) [46,47,48,49,50,51,52,53,54,55,56, 58, 59, 63,64,65,66, 69, 71, 74,75,76,77, 80, 82] administered FLS according to international guidelines, 33 (89.2%) [46,47,48,49,50,51,52, 55,56,57,58,59,60,61,62, 64,65,66,67,68, 70,71,72,73,74,75,76,77,78,79,80,81,82] reported the practitioners involved in the FLS programs, 34 (91.9%) [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67, 69,70,71,72,73,74,75,76,77, 79, 80, 82] were rated as having a low risk of bias, and 22 (59.5%) [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] were meta-analyzed. Additionally, of the meta-analyzed studies, 17 (77.3%) [46,47,48,49,50,51,52,53,54,55,56, 58, 59, 63,64,65,66] administered FLS according to international guidelines, 12 (54.5%) [46,47,48,49, 52,53,54, 56, 59, 63, 65, 66] prescribed bisphosphonates as the first-line anti-osteoporotic treatment, and eight (36.4%) [46, 47, 55, 56, 64,65,66] had nurses as the FLS coordinators. Moreover, 35 (94.6%) studies [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63, 65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80, 82] followed-up participants for at least 1 year, with only one study [64] terminating follow-up at 6 months. Most of the studies included (23; 62.2% studies) [46,47,48,49,50,51, 55, 56, 60,61,62,63, 65, 66, 68, 69, 71, 72, 74, 76, 79,80,81] did not report how the follow-up was conducted. Of the 14 studies [52,53,54, 57,58,59, 64, 67, 70, 73, 75, 77, 78, 82] that reported how the follow-up data was obtained, nine studies (64.3%) [52,53,54, 58, 59, 64, 75, 77, 82] administered FLS according to international guidelines.
This review found low certainty evidence showing that the risk of secondary fragility fracture was lower in the FLS intervention compared to the non-FLS intervention at 1-year follow-up, and this outcome became steady at greater or equal to 2 years of follow-up with moderate certainty of the evidence. The treatment effects achieved in this review are also considered clinically important (RR > 1.25 or < 0.75) [42, 44] at both 1 year and greater or equal to 2 years of follow-ups. This compelling evidence is well-supported by recent systematic reviews and meta-analyses that have found an FLS to be effective in reducing secondary fragility fractures [16, 22, 30]. Contrary to our review, previous reviews did not appraise the certainty of the evidence, and neither did they interpret their findings based on clinical significance [16, 22, 30]. Additionally, these reviews were not progressively registered which indicates the weak features of the reviews. These findings indicate that our current review is unique and has established with moderate certainty the overall effect of an FLS in reducing the risk of secondary fragility fractures in older adults.
This review also found moderate certainty showing no significant difference between FLS and non-FLS interventions in the risk of secondary fragility fracture in the intermediate-term follow-up (< 1 year but ≥ 3 months). This finding underscored the significance of long-term follow-up data in FLS programs, and this has been demonstrated by the clinically relevant treatment effects found in this review at both 1 year and greater or equal to 2 years of follow-ups. Additionally, 35 (94.6%) studies [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63, 65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80, 82] in this review also followed-up participants for at least 1 year, with only one study [64] terminating follow-up at 6 months which further underscored the significance of long-term follow-up data. Fracture healing depends on the dynamic balance between bone formation and bone resorption, and any factor that changes this balance can affect the fracture healing time and prognosis of the injury [83]. Osteoporosis is considered a possible risk factor that can change the dynamic balance between bone formation and bone resorption leading to low BMD and micro-architectural deterioration of bone structure, resulting in fragility and an increased fracture risk [84]. Anti-osteoporotic drugs, especially bisphosphonates which are the first-line medications, can inhibit osteoclast activities resulting in decreased bone resorption which may have negative effects on bone remodeling, thereby prolonging fracture healing [83, 84]. Given that bone remodeling can take months to years before it can occur, long-term anti-osteoporotic therapies are, therefore, desirable. These findings are well-supported by previous reviews which highlighted the significance of long-term follow-up data for improving secondary fragility fractures [29, 35, 85, 86] and osteoporosis in the elderly [87, 88].
It is important to note that the majority of the included studies in this review administered FLS according to international guidelines with DEXA as the most common diagnostic tool for osteoporosis, bisphosphonates as the first-line anti-osteoporotic treatment for patients with normal renal function, and denosumab for patients with renal impairment or as a second-line treatment. These findings are consistent with the recently published guidelines for the prevention of fragility fractures and the management of osteoporosis [89,90,91]. Additionally, this review also found that most of the included studies (33, 89.2% studies) reported the practitioners involved in the FLS programs, with nurses being the most common FLS coordinators who were mostly supported by members of the multidisciplinary team including physicians, general practitioners, rheumatologists, endocrinologists, orthogeriatricians, orthopedic surgeons, traumatologists, laboratory scientists, or physiotherapists. These findings are similar to previous reviews which highlighted the significance of FLS coordinators and multidisciplinary approach to preventing secondary fragility fractures in older adults [6, 18, 24]. Contrary to our review, previous reviews [6, 18, 24] did not collate evidence about the impact of an FLS in reducing the risk of secondary fragility fracture which the current review was able to perform. These findings indicate that our current review would inform with moderate certainty the public, patients, and healthcare professionals about the effect of an FLS in reducing secondary fragility fracture, the types of the FLS, and the roles of various disciplines involved in the delivery of the FLS.
Funnel plots showed symmetrical distributions for both meta-analysis comparisons at the intermediate-term and greater or equal to 2 years of follow-ups. However, the small number of pooled studies (less than 10) at the intermediate-term follow-up indicates that this comparison could potentially be biased and was therefore downgraded. Additionally, the funnel plot for meta-analysis comparison at 1-year follow-up showed a lack of symmetry and was also downgraded due to publication bias. Moreover, the meta-analysis comparisons in this review showed some level of heterogeneity, and sensitivity analyses were performed. The sensitivity analyses (comprising both prospective and retrospective cohorts) supported the findings of the meta-analyses with moderate certainty of the evidence and no observed heterogeneity at all levels of follow-ups, indicating that these findings may be attributable to chance only. Although heterogeneity can be avoided to some extent, it can never be prevented completely [92]. Therefore, this review failed to objectively identify the reasons for the heterogeneity in the meta-analysis comparisons. However, we attributed this problem to differences in the treatment guidelines given that no more than two studies used the same guidelines.
Clinical implications of this systematic review
The findings of this systematic review suggest that guideline-based and non-guideline-based FLS programs combined showed a moderate significant reduction in secondary fragility fractures compared to usual care; however, the guideline-based FLS programs appeared to make more substantial progress in reducing secondary fragility fractures. This has been demonstrated by the subgroup analyses in this review which showed the lack of impact of removing non-guideline-based FLS interventions to alter the results of the initial meta-analyses at the intermediate-term (RR 0.99, CI 0.83 to 1.17, p = 0.87; four pooled studies) [48, 53, 58, 64], long-term at 1 year (RR 0.23, CI 0.11 to 0.50, p = 0.0002; five pooled studies) [46, 47, 58, 65, 66], and long-term at ≥ 2 years (RR 0.63, CI 0.48 to 0.83, p = 0.00008; nine pooled studies) [49,50,51,52, 54,55,56, 59, 63]. Evidence-based guidelines provide synthesized evidence and standardized recommendations and protocols for the establishment and operation of FLS programs with considerations for regional differences and organizational variations. Of the 25 (67.6%) studies [46,47,48,49,50,51,52,53,54,55,56, 58, 59, 63,64,65,66, 69, 71, 74,75,76,77, 80, 82] that administered interventions according to international guidelines, 19 studies (76.0%) [46,47,48,49,50,51,52, 58, 59, 64,65,66, 71, 74,75,76,77, 80, 82], reported the practitioners involved in the FLS programs, with 11 studies (44.0%) [46, 47, 55, 56, 64,65,66, 74,75,76,77, 82], reporting nurses as the FLS coordinators. Additionally, 19 (76.0%) studies [46,47,48,49, 52,53,54, 56, 59, 63, 65, 66, 71, 74,75,76,77, 82], also prescribed bisphosphonates as anti-osteoporotic medications, with 9 (36.0%) studies prescribing denosumab [46,47,48,49, 53, 59, 74, 77, 82] and teriparatide [46,47,48, 53, 59, 63, 73, 74, 77] each as the anti-osteoporotic medications. These findings indicate that the included studies were similar in their programs’ administration. However, it is also evident that the characteristics of the FLS are not homogenous in the reviewed studies making it challenging to determine which FLS characteristics are likely to result in the most clinical effectiveness. The primary characteristics broadly included (1) identification of patients with fragility fracture when patients access hospital care; (2) evaluation of risk using different tests and/or procedures; (3) establishment of a diagnosis, treatment recommendations, and/or initiation; (4) outpatient follow-up either with the FLS or referral to primary care doctor; and finally, (5) education and communication with the patient’s physician at handover from the FLS to the primary care physician. Risk identification commonly included assessments such as previous medical history and medication exposure, fall risk, mobility, physical activity, tobacco use, nutrition, blood serum vitamin D and calcium, fracture risk, and bone density measurement. The process of identifying patients at risk for secondary fracture varied based on the health system; for example, Inderjeeth et al. [58] described the use of an emergency department information system (EDIS) to identify patients with a minimal trauma fracture prior to discharge so as not to compete with emergency treatment the patient would be receiving (type-C FLS). In contrast, Eekman et al. [70] referred patients with minimal trauma fracture to the FLS directly from the Emergency department (type-B FLS). However, type-A FLS, which identifies, investigates, and initiates treatment without a referral to primary care physicians, reported by most of the reviewed studies (25, 67.6%) [46, 47, 50,51,52,53,54,55,56,57, 60, 62,63,64, 66, 67, 69, 70, 73, 74, 77,78,79,80,81] is considered best practice [1, 5, 6, 29] and was used by 17 (45.9%) studies [46, 47, 50,51,52,53,54,55,56, 59, 63, 64, 66, 69, 74, 77, 80, 82] that administered FLS according to international guidelines [46,47,48,49,50,51,52,53,54,55,56, 58, 59, 63,64,65,66, 69, 71, 74,75,76,77, 80, 82].
A key feature of the FLS programs is the coordinator role. Early Identification and assessment are critical first steps to ensure that the patient who has sustained a fragility fracture is promptly evaluated to facilitate the initiation of evidence-based treatment. Equally important are the clinicians who are part of the FLS because they need to ensure that every patient at risk receives the comprehensive assessment needed. A multidisciplinary team of clinicians with knowledge about osteoporosis risk and management can collaborate effectively to ensure that a comprehensive risk assessment is conducted, a tailored patient-centered treatment plan is initiated, and that the patient receives sufficient follow-up care. Follow-up care at 2 years and longer is the most ideal given the antiresorptive function of the osteoporotic medications, the time taken for bone remodeling to occur and the pooled treatment effects of greater than 0.5 (clinically important) [42, 44] at ≥ 2 years obtained in this current review at the initial meta-analysis, sensitivity analysis, and sub-group analysis stages. These current findings have been supported by a recent review which reported a 70.0% lower probability (odds ratio 0.70, CI 0.52 to 0.93, p = 0.01) of subsequent fractures with FLS care versus non-FLS care with the most significant benefit being demonstrated in studies with more than 2 years of follow-up (odds ratio 0.57, CI 0.34 to 0.94, p = 0.03) compared to those with less than or equals to 2 years of follow-up (odds ratio 0.73, CI 0.51 to 1.03, p = 0.07) [22, 35].
In this review, 57.0% of FLS employed nurses in their programs. A systematic review examining the role and impact of advanced practice nurses (APNs) in caring for patients with fragility hip fracture found that advanced practice nurses are optimally positioned to coordinate or manage clinical pathways or protocols post-fragility hip fracture [93]. The review identified six characteristics of APNs based on 18 studies that matched inclusion criteria with a total of 43,218 participants post-hip-fracture including (1) coordination; (2) collaboration; (3) education; (4) assessment, investigation, and treatment recommendations; (5) discharge planning, support, and follow up; and (6) documentation. The review found that overall mortality and length of stay improved in patients with fragility hip fractures when characteristics of the APN roles were present [93]. In this current review, the use of nurses was evident, with nurse coordinators of FLS [52, 57, 73, 82] and nurse-practitioner-led FLS [64, 74] not only coordinating the FLS while the patient was in the care of the FLS but also communicating with the patient’s primary care provider during handover. However, patient compliance with the treatment plan and follow-up with their primary care physician could have an impact on sustained outcomes [30]. In addition, patient co-morbidities and general health are important considerations regarding how effective the FLS program may be in the long term [30].
To optimize muscle mass, BMD, and functional mobility, the evidence-based guidelines for osteoporosis, and fracture prevention recommend a multifaceted and targeted approach that also includes basic weight-bearing activities and exercise such as walking [94]. However, we found that while all studies included in this review used pharmacologic interventions in their treatment plan, only a few studies on hip fractures [46, 47, 53, 54, 80], non-vertebral fractures [55], or fractures of any location [57, 64, 70] discussed their use of physical therapy [53,54,55, 57, 64, 70] and rehabilitation [46, 47, 80] as part of their treatment plan. In addition, very few papers mentioned completing a nutritional assessment and providing education on dietary changes [46,47,48,49, 59, 82]. However, studies show that there is a positive association between dietary patterns and the impact on bone health. In a scoping review of 49 relevant studies conducted in over 20 countries and published between 2002 and June 2016 that examined various outcomes, including bone mineral density (BMD), bone biomarkers, osteoporosis, and fracture incidence found that adopting healthy dietary patterns, that emphasized the intake of fruit, vegetables, whole grains, poultry and fish, nuts and legumes, and low-fat dairy products and de-emphasized the intake of soft drinks, fried foods, meat and processed products, sweets and desserts, and refined grains showed a beneficial impact on bone health and decrease osteoporosis and fracture risk [95]. While prevention is always better than cure, recommendations to address modifiable risk factors such as diet and physical activity may augment the pharmacologic intervention aimed at reducing the risk for secondary fragility fractures [96].
Strengths and limitations of the reviewed studies and recommendations for future research
Most of the studies included in this review (25, 67.6% studies) administered FLS according to international guidelines and reported the practitioners involved in the FLS programs. Additionally, most of the included studies used DEXA for the diagnosis of osteoporosis (22; 59.5% studies), prescribed bisphosphonates as the first-line anti-osteoporotic treatment (23, 62.2% studies), and denosumab as the second-line treatment (10, 27.0% studies) which conformed with international guidelines [89,90,91]. However, it is important to note that strontium ranelate (received by 48 participants) was removed from the market [97], and also, denosumab (received by 544 participants) is a more recent drug which was introduced in 2005 [71] compared to bisphosphonate (received by 9823 participants) which was introduced in the 1960s [98]. Therefore, the timing of the data collection would have also influenced the findings of this review. Most of the studies included (23; 62.2% studies) [46,47,48,49,50,51, 55, 56, 60,61,62,63, 65, 66, 68, 69, 71, 72, 74, 76, 79,80,81] did not report how the follow-up data was obtained which could be improved in future studies. The studies included also have methodological quality limitations including failure to recruit controls (non-exposed or non-treated cohorts), failure to match cohorts (exposed or treated participants) with controls to have the same or similar characteristics, such as age, sex, fracture types, and covariates with a large number of values or levels, such as area of residence (e.g., post code) and clinics/hospitals, failure to adjust for confounders (e.g., age, sex, co-morbidities, or covariates) in the analysis, failure to account for the enrolled participants, failure to blind treating practitioners, failure to blind participants, and failure to blind outcome assessors. Although it is not possible to recruit controls or match cohorts with controls in retrospective studies, it is possible to adjust for confounders in the analysis and account for the enrolled participants in the study [99]. While it is also difficult to blind practitioners and patients to osteoporosis treatment, particularly in the case of fragility fractures, the outcome assessors can be blinded [100]. Another limitation of the included studies is the use of different guidelines for the management of osteoporosis which may indicate the reason for the heterogeneity found in the meta-analysis comparisons in this review. Future studies should use guideline-based FLS interventions to ensure consistency and effectiveness of treatment, match cohorts with controls for equivalence of data, adjust for confounders in the analysis to ensure trustworthiness of data, and account for the enrolled participants in their studies to minimize attrition [100].
Strengths and limitations of the current review and recommendations for future research
Although the previous reviews [16, 22, 30] collated data on secondary fragility fractures, these reviews were not prospectively registered, did not appraise the certainty of the evidence, and did not interpret their findings based on clinical significance which the current review was able to perform. Additionally, systematic reviews are of paramount importance when meta-analyses are performed [34]. The results of meta-analyses can improve the precision of estimates of effect, answer questions not posed by the individual studies, settle controversies arising from apparently conflicting studies, and generate new hypotheses [34]. This current review performed meta-analyses (for eligible studies) which were further confirmed by sensitivity and sub-group analyses of the retrieved evidence. Moreover, the nature of the FLS and the roles of the various disciplines involved in the delivery of the FLS were also reported in this review. These significant factors indicate that the outcomes of the current systematic review are robust and represent for the first time, the best evidence synthesis on the overall effectiveness of an FLS in reducing the risk of secondary fragility fractures in older adults. The limitation of the current review is that it only included secondary fragility fracture as an outcome and failed to include other relevant outcomes (such as mortality, treatment initiation, and adherence) that can improve the overall impact of an FLS. In addition, due to the influence of other confounding variables such as fracture types, level of patient frailty prior to the fracture, co-morbidities, and treatment adherence, this current systematic review could not examine which specific components of the FLS made the FLS “effective”. Moreover, it is also not known if extra services such as fall prevention strategies were available to patients independently outside of the FLS, and if this was the case, then, they could have influenced the re-fracture rates which this review could not find out. Another limitation of the current review was the exclusion of trials not published in English. Although this problem may not be unrelated to insufficient funding and resources, it is important to note that excluding studies not published in English may not affect the overall outcomes of a systematic review [101]. However, we could not determine this definitively in our systematic review. Future reviews may, therefore, be conducted to address these limitations. Organizations that have implemented FLS should regularly evaluate the FLS program and include the multidisciplinary team’s role in their publications. Researchers that study the implementation of FLS to reduce secondary fragility fractures should provide detailed information about the FLS program including the exact characteristics and the processes from enrollment into the FLS to follow-up. Providing unique organization-related details provides important context. Including more specific details about the characteristics of the FLS would provide opportunities for a more robust analysis to determine the factors that positively impact secondary fracture prevention.
Conclusion
This review found clinically important low certainty evidence showing that the risk of secondary fragility fracture was lower in the FLS intervention compared to the non-FLS intervention at 1-year follow-up, and this outcome became steady at greater or equal to 2 years with moderate certainty of the evidence. Moreover, this review also found nurses to be the most common FLS coordinators, DEXA as the most common diagnostic tool for osteoporosis, bisphosphonates as the first-line anti-osteoporotic treatment, and denosumab as the second-line treatment. However, it is important to note that denosumab is a more recent drug which was introduced compared to bisphosphonate; therefore, the timing of the data collection would have also influenced the findings of this systematic review.
References
National Institute for Health and Care Excellence (NICE) (2012) WH. Osteoporosis: assessing the risk of fragility fracture clinical guide [CG146]. https://www.nice.org.uk/guidance/cg146/chapter/introduction#ftn.footnote_3. Accessed Jan 12, 2022
Kanis JA, Norton N, Harvey NC et al (2021) SCOPE 2021: a new scorecard for osteoporosis in Europe. Arch Osteoporos 16(1):82
Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12(5):417–427
Australian Institute of Health and Welfare (AIHW) (2018) AIoHa. Hip fracture incidence and hospitalisations in Australia 2015–16. https://www.aihw.gov.au/reports/injury/hip-fracture-incidence-in-australia-2015-16. Accessed Feb 20, 2021
International Osteoporosis Foundation Fracture Working Group (2021) Broken bones, broken lives: a roadmap to solve the fragility fracture crisis in the United Kingdom. United Kingdom
Osuna PM, Ruppe MD, Tabatabai LS (2017) Fracture liaison services: multidisciplinary approaches to secondary fracture prevention. Endocr Pract 23(2):199–206
van Oostwaard M (2018) Osteoporosis and the nature of fragility fracture: an overview. 2018 Jun 16. In: Hertz K, Santy-Tomlinson J, editors. Fragility fracture nursing: holistic care and management of the orthogeriatric patient [Internet]. Cham (CH): Springer. Chapter 1. https://www.ncbi.nlm.nih.gov/books/NBK543829/. https://doi.org/10.1007/978-3-319-76681-2_1
Hernlund E, et al (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 8: p 136
Mitchell PJ, Chan DD, Lee JK, Tabu I, Alpuerto BB (2022) The global burden of fragility fractures - what are the differences, and where are the gaps. Best Pract Res Clin Rheumatol 36(3):101777. https://doi.org/10.1016/j.berh.2022.101777
Cheung CL, Ang SB, Chadha M, Chow ES, Chung YS, Hew FL, Jaisamrarn U, Ng H, Takeuchi Y, Wu CH, Xia W, Yu J, Fujiwara S (2018) An updated hip fracture projection in Asia: the Asian Federation of Osteoporosis Societies study. Osteoporos Sarcopenia 4(1):16–21. https://doi.org/10.1016/j.afos.2018.03.003
Lesnyak O, Bilezikian JP, Zakroyeva A (2020) Working group for the audit on burden of osteoporosis in Eurasian region. Report on the audit on burden of osteoporosis in eight countries of the Eurasian region: Armenia, Belarus, Georgia, Moldova, Kazakhstan, the Kyrgyz Republic, the Russian Federation, and Uzbekistan. Arch Osteoporos 15(1):175. https://doi.org/10.1007/s11657-020-00836-y
Aziziyeh R, Amin M, Habib M, Garcia Perlaza J, Szafranski K, McTavish RK, Disher T, Lüdke A, Cameron C (2019) The burden of osteoporosis in four Latin American countries: Brazil, Mexico, Colombia, and Argentina. J Med Econ 22(7):638–644. https://doi.org/10.1080/13696998.2019.1590843
Hansen D, Bazell C, Pelizzari P, Pyenson B (2019) Medicare cost of osteoporotic fractures. The clinical and cost burden of an important consequence of osteoporosis. Milliman research report, commissioned by the National Osteoporosis Foundation
International Osteoporosis Foundation (2022) IOF capture the fracture® map of best practice. Available from: https://www.capturethefracture.org/map-of-best-practice. Accessed 7th July 2023
Akesson KE, McGuigan FEA (2021) Closing the osteoporosis care gap. Curr Osteoporos Rep 19(1):58–65
Barton DW, Piple AS, Smith CT, Moskal SA, Carmouche JJ (2021) The clinical impact of fracture liaison services: a systematic review. Geriatric Orthop Surg Rehabil 12
Briot K (2017) Fracture Liaison Services. Curr Opin Rheumatol 29(4):416-421
Cha YH, Ha YC, Park KS, Yoo JI (2020) What is the role of coordinators in the secondary fracture prevention program? J Bone Metab 27(3):187–199
de Bruin IJA, Wyers CE, van den Bergh JPW, Geusens PPMM (2017) Fracture liaison services: do they reduce fracture rates? Ther Adv Musculoskeletal Dis 9(7):157–164
Ganda K, Mitchell PJ, Seibel MJ (2018) Models of secondary fracture prevention: systematic review and metaanalysis of outcomes. In: Secondary fracture prevention: an international perspective 33–62
Leslie WD, Crandall CJ (2019) Population-based osteoporosis primary prevention and screening for quality of care in osteoporosis, current osteoporosis reports. Curr Osteoporos Rep 17(6):483–490
Li N, Hiligsmann M, Boonen A et al (2021) The impact of fracture liaison services on subsequent fractures and mortality: a systematic literature review and meta-analysis. Osteoporos Int 32(8):1517–1530. https://doi.org/10.1007/s00198-021-05911-9
Naranjo A, Molina A, Sepulveda C, Rubino FJ, Martin N, Ojeda S (2020) The evolution of an FLS in search of excellence: the experience of Gran Canaria. Arch Osteoporos 15(1):108
Pape HC, Bischoff-Ferrari HA (2017) How can we influence the incidence of secondary fragility fractures? a review on current approaches. Injury 48:S24–S26
Pioli G, Bendini C, Pignedoli P, Giusti A, Marsh D (2018) Orthogeriatric co-management – managing frailty as well as fragility. Injury 49(8):1398–1402
Sáez-López P, Etxebarria-Foronda I, Mesa Lampre MP, Alonso García N, Sánchez HN (2019) Efficacy, cost, and aspects to take into account in the treatment of osteoporosis in the elderly. Revista Espanola de Geriatria y Gerontologia 54(3):156–167
Schweser KM, Crist BD (2017) Osteoporosis: a discussion on the past 5 years. Curr Rev Musculoskelet Med 10(2):265–274
Shibli-Rahhal A (2018) Secondary prevention of low-trauma fractures: in search of an effective solution. J Clin Outcomes Manag 25(4):177–185
Walters S, Khan T, Ong T, Sahota O (2017) Fracture liaison services: improving outcomes for patients with osteoporosis. Clin Interv Aging 12:117–127
Wu CH, Chen CH, Chen PH et al (2018) Identifying characteristics of an effective fracture liaison service: systematic literature review. Osteoporos Int 29(5):1023–1047
Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, Stewart L (2012) The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev 9(1):2. https://doi.org/10.1186/2046-4053-1-2
Brooke BS, Schwartz TA, Pawlik TM (2021) MOOSE reporting guidelines for meta-analyses of observational studies. JAMA Surg 156(8):787–788. https://doi.org/10.1001/jamasurg.2021.0522
Kwon Y, Lemieux M, McTavish J, Wathen N (2015) Identifying and removing duplicate records from systematic review searches. J Med Libr Assoc 103(4):184–188. https://doi.org/10.3163/1536-5050.103.4.004
Higgins JPT, Thomas J, Chandler J, et al. (editors) (2020) Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane
Ganda K (2021) Fracture liaison services: past, present and future. Osteoporos Int 32:1461–1464. https://doi.org/10.1007/s00198-021-05982-8
Wells GA, Shea B,O’Connell D, Peterson J,Welch V, Losos PTM. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605. https://doi.org/10.1007/s10654-010-9491-z
Cashin AG, McAuley JH (2020) Clinimetrics: physiotherapy evidence database (PEDro) scale. J Physiother 66(1):59. https://doi.org/10.1016/j.jphys.2019.08.005
Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al (2011) GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol 64:407–415. https://doi.org/10.1016/j.jclinepi.2010.07.017
Guyatt GH, Oxman AD, Kunz R, et al (2011) GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol 64:1294–1302. https://doi.org/10.1016/j.jclinepi.2011.03.017
Guyatt GH, Oxman AD, Kunz R, et al (2011) GRADE guidelines: 8. Rating the quality of evidence--indirectness. J Clin Epidemiol 64(12):1303–10. https://doi.org/10.1016/j.jclinepi.2011.04.014
Guyatt GH, Oxman AD, Kunz R, et al (2011) GRADE guidelines 6. Rating the quality of evidence--imprecision. J Clin Epidemiol 64(12):1283–1293. https://doi.org/10.1016/j.jclinepi.2011.01.012
Guyatt GH, Oxman AD, Montori V, et al (2011) GRADE guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol 64:1277–1282. https://doi.org/10.1016/j.jclinepi.2011.01.011
Kulig M, Perleth M, Langer G, et al (2012) GRADE guidelines: 6. Rating the quality of evidence: imprecision. Z Evid Fortbild Qual Gesundhwes 106:677–688. https://doi.org/10.1016/j.zefq.2012.10.016
Harrer M, Cuijpers P, Furukawa TA, Ebert DD (n.d.) Doing meta-analysis with R: a hands-on guide. Boca Raton, FL and London: Chapman & Hall/CRC Press. ISBN 978–0–367–61007–4
Amphansap T, Stitkitti N, Dumrongwanich P (2016) Evaluation of Police General Hospital’s fracture liaison service (PGH’s FLS): the first study of a fracture liaison service in Thailand. Osteoporos Sarcopenia 2(4):238–243. https://doi.org/10.1016/j.afos.2016.09.002
Amphansap T, Stitkitti N, Arirachakaran A (2020) The effectiveness of Police General Hospital’s fracture liaison service (PGH’s FLS) implementation after 5 years: a prospective cohort study. Osteoporos Sarcopenia 6(4):199–204. https://doi.org/10.1016/j.afos.2020.11.004
Axelsson KF, Jacobsson R, Lund D, Lorentzon M (2016) Effectiveness of a minimal resource fracture liaison service. Osteoporos Int 27(11):3165–3175. https://doi.org/10.1007/s00198-016-3643-2
Axelsson KF, Johansson H, Lundh D, Möller M, Lorentzon M (2020) Association between recurrent fracture risk and implementation of fracture liaison services in four Swedish hospitals: a cohort study. J Bone Miner Res 35(7):1216–1223. https://doi.org/10.1002/jbmr.3990
Bachour F, Rizkallah M, Sebaaly A, Barakat A, Razzouk H, El Hage R, Nasr R, El Khoury M, Maalouf G (2017) Fracture liaison service: report on the first successful experience from the Middle East. Arch Osteoporos 12(1):79. https://doi.org/10.1007/s11657-017-0372-x
Chien LN, Li YF, Yang RS, Yang TH, Chen YH, Huang WJ, Tsai HY, Li CY, Chan DC (2022) Real-world cost-effectiveness analysis of the fracture liaison services model of care for hip fracture in Taiwan. J Formos Med Assoc 121(1 Pt 2):425–433. https://doi.org/10.1016/j.jfma.2021.05.028
Davidson E, Seal A, Doyle Z, Fielding K, McGirr J (2017) Prevention of osteoporotic refractures in regional Australia. Aust J Rural Health 25(6):362–368. https://doi.org/10.1111/ajr.12355
González-Quevedo D, Bautista-Enrique D, Pérez-Del-Río V, Bravo-Bardají M, García-de-Quevedo D, Tamimi I (2019) Fracture liaison service and mortality in elderly hip fracture patients: a prospective cohort study. Osteoporos Int 31(1):77–84. https://doi.org/10.1007/s00198-019-05153-w
González-Quevedo D, Pérez-Del-Río V, Moriel-Garceso D, Fernández-Arroyabe N, García-Meléndez G, Montañez-Ruiz M, Bravo-Bardají M, García-de-Quevedo D, Tamimi I (2022) A 2-year follow-up of a novel fracture liaison service: can we reduce the mortality in elderly hip fracture patients? a prospective cohort study. Osteoporos Int 31:1–8. https://doi.org/10.1007/s00198-022-06298-x
Huntjens KM, van Geel TC, Geusens PP, Winkens B, Willems P, van den Bergh J, Brink PR, van Helden S (2011) Impact of guideline implementation by a fracture nurse on subsequent fractures and mortality in patients presenting with non-vertebral fractures. Injury 42(Suppl 4):S39-43. https://doi.org/10.1016/S0020-1383(11)70011-0
Huntjens KM, van Geel TA, van den Bergh JP, van Helden S, Willems P, Winkens B, Eisman JA, Geusens PP, Brink PR (2014) Fracture liaison service: impact on subsequent nonvertebral fracture incidence and mortality. J Bone Joint Surg Am 96(4):e29. https://doi.org/10.2106/JBJS.L.00223
Inácio AM, Marques LLM, Borba VZC, Moreira CA (2022) Incidence of fractures and clinical profile of patients following up at a fracture liaison service in the city of Curitiba. Aging Clin Exp Res 1:1–7. https://doi.org/10.1007/s40520-022-02116-w
Inderjeeth CA, Raymond WD, Briggs AM, Geelhoed E, Oldham D, Mountain D (2018) Implementation of the Western Australian osteoporosis model of care: a fracture liaison service utilising emergency department information systems to identify patients with fragility fracture to improve current practice and reduce re-fracture rates: a 12-month analysis. Osteoporos Int 29(8):1759–1770. https://doi.org/10.1007/s00198-018-4526-5
Mugnier B, Daumas A, Couderc AL, Mizzi B, González T, Amrani A, Lévêque P, Aymes B, Argenson JN, Villani P (2019) Clinical effectiveness of osteoporosis treatment in older patients: a fracture liaison service-based prospective study. J Women Aging 31(6):553–565. https://doi.org/10.1080/08952841.2018.1529473
Nakayama A, Major G, Holliday E, Attia J, Bogduk N (2016) Evidence of effectiveness of a fracture liaison service to reduce the re-fracture rate. Osteoporos Int 27(3):873–879. https://doi.org/10.1007/s00198-015-3443-0
Osaki M, Okuda R, Saeki Y, Okano T, Tsuda K, Nakamura T, Morio Y, Nagashima H, Hagino H (2021) Efficiency of coordinator-based osteoporosis intervention in fragility fracture patients: a prospective randomized trial. Osteoporos Int 32(3):495–503. https://doi.org/10.1007/s00198-021-05825-6
Sanli I, van Helden SH, Ten Broeke RHM, Geusens P, Van den Bergh JPW, Brink PRG, Poeze M (2019) The role of the fracture liaison service (FLS) in subsequent fracture prevention in the extreme elderly. Aging Clin Exp Res 31(8):1105–1111. https://doi.org/10.1007/s40520-018-1054-2
Shin YH, Hong WK, Kim J, Gong HS (2020) Osteoporosis care after distal radius fracture reduces subsequent hip or spine fractures: a 4-year longitudinal study. Osteoporos Int 31(8):1471–1476. https://doi.org/10.1007/s00198-020-05410-3
Singh S, Whitehurst DG, Funnell L, Scott V, MacDonald V, Leung PM, Friesen K, Feldman F (2019) Breaking the cycle of recurrent fracture: implementing the first fracture liaison service (FLS) in British Columbia, Canada. Arch Osteoporos 14(1):116. https://doi.org/10.1007/s11657-019-0662-6
Sorensen A, Gimarc D, Bice M, Hare K, Anderson PA, Ross A (2021) Improving secondary fracture prevention after vertebroplasty: implementation of a fracture liaison service. J Am Coll Radiol 18(9):1235–1238. https://doi.org/10.1016/j.jacr.2021.06.004
Van der Kallen J, Giles M, Cooper K, Gill K, Parker V, Tembo A, Major G, Ross L, Carter J (2014) A fracture prevention service reduces further fractures two years after incident minimal trauma fracture. Int J Rheum Dis 17(2):195–203. https://doi.org/10.1111/1756-185X.12101
Wasfie T, Jackson A, Brock C, Galovska S, McCullough JR, Burgess JA (2019) Does a fracture liaison service program minimize recurrent fragility fractures in the elderly with osteoporotic vertebral compression fractures? Am J Surg 217(3):557–560. https://doi.org/10.1016/j.amjsurg.2018.09.027
Benzvi L, Gershon A, Lavi I, Wollstein R (2016) Secondary prevention of osteoporosis following fragility fractures of the distal radius in a large health maintenance organization. Arch Osteoporos 11:20. https://doi.org/10.1007/s11657-016-0275-2
Briggs AM, Sun W, Miller LJ, Geelhoed E, Huska A, Inderjeeth CA (2015) Hospitalisations, admission costs and re-fracture risk related to osteoporosis in Western Australia are substantial: a 10-year review. Aust N Z J Public Health 39(6):557–562. https://doi.org/10.1111/1753-6405.12381
Eekman DA, van Helden SH, Huisman AM, Verhaar HJ, Bultink IE, Geusens PP, Lips P, Lems WF (2014) Optimizing fracture prevention: the fracture liaison service, an observational study. Osteoporos Int 25(2):701–709. https://doi.org/10.1007/s00198-013-2481-8
Hawley S, Leal J, Delmestri A, Prieto-Alhambra D, Arden NK, Cooper C, Javaid MK, Judge A; REFReSH Study Group (2016a) Anti-osteoporosis medication prescriptions and incidence of subsequent fracture among primary hip fracture patients in England and Wales: an interrupted time-series analysis. J Bone Miner Res 31(11):2008–2015. https://doi.org/10.1002/jbmr.2882
Hawley S, Javaid MK, Prieto-Alhambra D, Lippett J, Sheard S, Arden NK, Cooper C, Judge A; REFReSH study group (2016b) Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age ageing 45(2):236–42. https://doi.org/10.1093/ageing/afv204
Kim D, Mackenzie D, Cutfield R (2016) Implementation of fracture liaison service in a New Zealand public hospital: Waitemata district health board experience. N Z Med J 129(1445):50–55
Lüthje P, Nurmi-Lüthje I, Tavast N, Villikka A, Kataja M (2021) Evaluation of minimal fracture liaison service resource: costs and survival in secondary fracture prevention-a prospective one-year study in South-Finland. Aging Clin Exp Res 33(11):3015–3027. https://doi.org/10.1007/s40520-021-01826-x
Majumdar SR, Lier DA, Hanley DA, Juby AG, Beaupre LA; STOP-PRIHS Team (2017) Economic evaluation of a population-based osteoporosis intervention for outpatients with non-traumatic non-hip fractures: the “catch a break” 1i [type C] FLS. Osteoporos Int 28(6):1965–1977. https://doi.org/10.1007/s00198-017-3986-3
Majumdar SR, Lier DA, McAlister FA, Johnson JA, Rowe BH, Beaupre LA (2019) Cost-effectiveness of osteoporosis interventions to improve quality of care after upper extremity fracture: results from a randomized trial (C-STOP trial). J Bone Miner Res 34(7):1220–1228. https://doi.org/10.1002/jbmr.3699
Sánchez MA, Segura JE, Alajmo G, Nossa JM, Correa A, Leal E, Moscoso A, Pineda GA, Aya AC (2020) Implementation of a postfracture care program in a private hospital in Colombia. J Osteoporos 18(2020):8208397. https://doi.org/10.1155/2020/8208397
Shimodan S, Sato D, Takahashi K, Nakamura Y, Hyakkan R, Watanabe T, Hishimura R, Ota M, Shimizu H, Hojo Y, Hasegawa Y, Chubachi T, Yasui K, Tsujimoto T, Tsukuda Y, Asano T, Takahashi D, Takahata M, Iwasaki N, Shimizu T (2020) Ten years change in post-fracture care for hip fracture patients. J Bone Miner Metab 38(2):222–229. https://doi.org/10.1007/s00774-019-01047-3
Solomon DH, Patrick AR, Schousboe J, Losina E (2014) The potential economic benefits of improved postfracture care: a cost-effectiveness analysis of a fracture liaison service in the US health-care system. J Bone Miner Res 29(7):1667–1674. https://doi.org/10.1002/jbmr.2180
Suzuki N, Arai K, Kon S, Yamanaka K, Otsuka H, Koizumi M, Hosaka N, Tsuchiya M, Mochizuki T, Kuraishi T, Murayama T, Tashi H, Oike N, Wakasugi M, Takahashi Y, Nakadai M, Endo N (2017) Challenges to prevent secondary fractures in patients with hip fractures in Joetsu Myoko, Japan through the increased use of osteoporosis treatment and collaboration with family doctors. J Bone Miner Metab 35(3):315–323. https://doi.org/10.1007/s00774-016-0758-7
Vrignaud A, Pelletier S, Dernis E, Moui Y, Haettich B (2018) Improvement in the primary and secondary prevention of osteoporosis by a fracture liaison service: feedback from a single French center care pathway. Arch Osteoporos 13(1):110. https://doi.org/10.1007/s11657-018-0523-8
Yates CJ, Chauchard MA, Liew D, Bucknill A, Wark JD (2015) Bridging the osteoporosis treatment gap: performance and cost-effectiveness of a fracture liaison service. J Clin Densitom 18(2):150–6. https://doi.org/10.1016/j.jocd.2015.01.003
Gao Y, Liu X, Gu Y et al (2021) The effect of bisphosphonates on fracture healing time and changes in bone mass density: a meta-analysis. Front Endocrinol (Lausanne) 30(12):688269. https://doi.org/10.3389/fendo.2021.688269
Gorter EA, Reinders CR, Krijnen P, Appelman-Dijkstra NM, Schipper IB (2021) The effect of osteoporosis and its treatment on fracture healing a systematic review of animal and clinical studies. Bone Rep 16(15):101117. https://doi.org/10.1016/j.bonr.2021.101117
Dyer SM, Crotty M, Fairhall N et al (2016) Fragility Fracture Network (FFN) Rehabilitation Research Special Interest Group. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr 16(1):158. https://doi.org/10.1186/s12877-016-0332-0
Adami G, Fassio A, Gatti D, et al (2022) Osteoporosis in 10 years’ time: a glimpse into the future of osteoporosis. Ther Adv Musculoskelet Dis 14. https://doi.org/10.1177/1759720X221083541
Anastasilakis AD, Toulis KA, Polyzos SA, Anastasilakis CD, Makras P (2012) Long-term treatment of osteoporosis: safety and efficacy appraisal of denosumab. Ther Clin Risk Manag 8:295–306. https://doi.org/10.2147/TCRM.S24239
Langdahl BL (2019) Management of endocrine disease: treatment breaks in long-term management of osteoporosis. Eur J Endocrinol 180(1):R29–R35. https://doi.org/10.1530/EJE-18-0282
Conley RB, Adib G, Adler RA et al (2020) Secondary fracture prevention: consensus clinical recommendations from a multistakeholder coalition. J Bone Miner Res 35(1):36–52. https://doi.org/10.1002/jbmr.3877
Gregson CL, Armstrong DJ, Bowden J, Cooper C, Edwards J, Gittoes NJL, Harvey N, Kanis J, Leyland S, Low R, McCloskey E, Moss K, Parker J, Paskins Z, Poole K, Reid DM, Stone M, Thomson J, Vine N, Compston J (2022) UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos 17(1):58. https://doi.org/10.1007/s11657-022-01061-5
Qaseem A, Hicks LA, Etxeandia-Ikobaltzeta I, Shamliyan T, Cooney TG; Clinical Guidelines Committee of the American College of Physicians (2023) Pharmacologic treatment of primary osteoporosis or low bone mass to prevent fractures in adults: a living clinical guideline from the American College of Physicians. Ann Intern Med. https://doi.org/10.7326/M22-1034
Melsen WG, Bootsma MC, Rovers MM, Bonten MJ (2014) The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect 20(2):123–129. https://doi.org/10.1111/1469-0691.12494
Allsop S, Morphet J, Lee S, Cook O (2021) Exploring the roles of advanced practice nurses in the care of patients following fragility hip fracture: a systematic review. J Adv Nurs 77(5):2166–2184. https://doi.org/10.1111/jan.14692
Dent E, Daly RM, Hoogendijk EO, Scott D (2023) Exercise to prevent and manage frailty and fragility fractures. Curr Osteoporos Rep 21(2):205–215. https://doi.org/10.1007/s11914-023-00777-8
Movassagh EZ, Vatanparast H (2017) Current evidence on the association of dietary patterns and bone health: a scoping review. Adv Nutr 8(1):1–16. https://doi.org/10.3945/an.116.013326
LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, Siris ES (2022) The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 33(10):2049–2102. https://doi.org/10.1007/s00198-021-05900-y. Epub 2022 Apr 28. Erratum in: Osteoporos Int. 2022
Strontium ranelate discontinued (2017) DTB 55(8): 86https://doi.org/10.1136/dtb.2017.8.0507
Widler L, Jaeggi KA, Glatt M, Müller K, Bachmann R, Bisping M, Born AR, Cortesi R, Guiglia G, Jeker H, Klein R, Ramseier U, Schmid J, Schreiber G, Seltenmeyer Y, Green JR (2002) Highly potent geminal bisphosphonates. From pamidronate disodium (Aredia) to zoledronic acid (Zometa). J Med Chem 45(17):3721–38. https://doi.org/10.1021/jm020819i
Belbasis L, Bellou V (2018) Introduction to epidemiological studies. Methods Mol Biol 1793:1–6. https://doi.org/10.1007/978-1-4939-7868-7_1
Sil A, Kumar P, Kumar R, Das NK (2019) Selection of control, randomization, blinding, and allocation concealment. Indian Dermatol Online J 10(5):601–605. https://doi.org/10.4103/idoj.IDOJ_149_19
Moher D, Pham B, Lawson ML, Klassen TP (2003) The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technol Assess 7(41):1–90. https://doi.org/10.3310/hta7410
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This systematic review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Danazumi, M.S., Lightbody, N. & Dermody, G. Effectiveness of fracture liaison service in reducing the risk of secondary fragility fractures in adults aged 50 and older: a systematic review and meta-analysis. Osteoporos Int 35, 1133–1151 (2024). https://doi.org/10.1007/s00198-024-07052-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-024-07052-1