Abstract
Summary
The impact of ROMO on the width of anabolic windows and the increase in BMD was reduced in the RA group compared to the non-RA group, and this reduction was associated with correlations to RA-related factors.
Purpose
To investigate the effects of romosozumab (ROMO) in postmenopausal osteoporosis, with and without comorbid rheumatoid arthritis (RA).
Methods
In this retrospective, case-controlled, multicenter study, 171 postmenopausal patients who did not receive oral glucocorticoid, comprising 59 in the RA group and 121 in the non-RA group, received uninterrupted ROMO treatment for 12 months. Propensity score matching was employed to ensure comparability in clinical backgrounds, resulting in 41 patients in each group. Baseline characteristics were as follows: overall (mean age, 76.3 years; T-score of lumbar spine (LS), − 3.0; 45.1% were treatment-naive for osteoporosis); RA group (anti-cyclic citrullinated peptide antibody (ACPA) positivity, 80.5%; titer, 206.2 U/ml; clinical disease activity index (CDAI), 13.6; health assessment questionnaire disability index (HAQ-DI), 0.9). Bone mineral density (BMD) and serum bone turnover markers were monitored over a 12-month period.
Results
The rate of increase in the bone formation marker, PINP, and the rates of decrease in the bone resorption marker, TRACP-5b, exhibited a trend toward smaller changes in the RA group compared to the non-RA group, implying a smaller anabolic window. After 12 months, the RA group displayed lower BMD increases in the LS (9.1% vs. 12.6%; P = 0.013) and total hip (2.4% vs. 4.8%; P = 0.025) compared to the non-RA group. Multiple regression analysis in the all RA group (n = 59) for the association between RA-specific factors and 12-month BMD changes revealed negative correlations between ACPA titer and LS BMD and between HAQ-DI and femoral neck BMD.
Conclusions
The efficacy of ROMO may be attenuated by RA-related factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) represents one of the principal etiologies of secondary osteoporosis [1], with decreased systemic bone mineral density (BMD) observed early in its course [2]. Various contributing factors to progressive bone loss in RA have been documented. Pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and IL-6, which are closely linked to disease activity, stimulate the expression of receptor activation of nuclear factor κB ligand (RANKL) by osteocytes, thereby promoting osteoclastogenesis [3]. Furthermore, TNF-α, glucocorticoid, and mechanical unloading also stimulate sclerostin production by osteocytes. Sclerostin, in turn, directly inhibits Wnt signaling, thus dampening osteoblast-mediated bone formation [4]. Additionally, sclerostin hinders the production of osteoprotegerin, an in vivo decoy receptor for RANKL produced by both osteocytes and osteoblasts, further exacerbating osteoclastogenesis [4]. Notably, individuals with RA exhibit higher levels of serum sclerostin and bone resorption markers compared to healthy controls [5], indicative of an augmented bone resorption and impaired bone formation in RA patients.
Romosozumab (ROMO), a monoclonal anti-sclerostin antibody, represents a novel therapeutic agent for osteoporosis. ROMO enhances Wnt signaling by neutralizing sclerostin [6]. By directly stimulating bone formation by osteoblasts and indirectly inhibiting bone resorption by osteoclasts [4], ROMO exerts a “dual effect,” resulting in a broader anabolic window when compared to other anti-osteoporosis agents [7]. Indeed, in patients with postmenopausal osteoporosis, ROMO has demonstrated superior improvements in BMD compared to alendronate, teriparatide [6], and denosumab (DMAb) [8].
However, it remains uncertain whether the altered bone metabolism seen in RA might potentiate or diminish the effects of ROMO. In this case-controlled study, our objective was to elucidate the impact of ROMO in RA patients compared to non-RA postmenopausal osteoporosis patients. Our primary endpoint was to assess differences in changes in bone turnover markers and BMD between the two groups, while our secondary endpoint aimed to elucidate the influence of RA-related factors on ROMO’s effects as determined by changes in BMD.
Methods
Study design and patients
This retrospective, case-controlled study was conducted across five centers. Treatment with ROMO (administered subcutaneously at 210 mg every month) was initiated between March 2019 and November 2021 for patients deemed to have a high fracture risk as defined by either the World Health Organization’s 1998 criteria or the Japanese Guidelines for Prevention and Treatment of Osteoporosis from 2011 [9]. Inclusion criteria encompassed patients with one or more of the following: (1) BMD T-score < − 2.5 with at least one fragility fracture, (2) lumbar spine (LS) BMD T-score < − 3.3, (3) two or more vertebral fractures, or (4) semiquantitative (SQ) grade 3 vertebral fracture [10].
Exclusion criteria for ROMO initiation were as follows: patients with contraindications to ROMO (i.e., those with major cardiovascular events within the past year), patients with conditions affecting bone metabolisms such as thyroid or parathyroid disorders, individuals receiving hormone replacement therapy, individuals with cancer undergoing radiation therapy involving the skeletal system, patients with osteomalacia (characterized by low serum levels of 25(OH)D, calcium, and phosphorus; elevated alkaline phosphatase; and intact parathyroid hormone), or patients with severe renal impairment (estimated glomerular filtration rate (eGFR) < 30 (ml/min/1.73 m2)). Additionally, patients lacking BMD data, male patients, and those with concurrent oral glucocorticoid use, other autoimmune diseases, or those who discontinued ROMO treatment before completing 12 months of therapy were also excluded from this study.
BMD assessment
BMD values of the LS (L2–L4), total hip (TH), and femoral neck (FN) were assessed using dual-energy X-ray absorptiometry (DXA) equipment (Horizon W; Hologic, Inc., Marlborough, MA, USA/PRODIGY; GE Healthcare, Tokyo, Japan) at baseline and subsequently at 6-month intervals following ROMO initiation. The percent coefficient of variation for L2-L4 was 0.63% for the Horizon system and 0.41% for the PRODIGY system. BMD data were standardized using the reference values obtained from the Japanese population for each respective DXA device, following the correction method proposed by the Japan Osteoporosis Society and the International Society for Clinical Densitometry Guidance [11]. As previously mentioned, regions of severe sclerosis, degenerative spine conditions, vertebral fractures, and surgical sites were excluded from the BMD measurements [12].
Biochemical markers of bone turnover
Blood samples were collected in the morning following an overnight fast. Bone turnover markers were measured at baseline and subsequently at 1, 6, and 12 months during the ROMO treatment. Total N-terminal type I procollagen propeptide (PINP; interassay coefficient of variation ≤ 5.0%; Roche Diagnostics, Basel, Switzerland) served as a bone formation marker, and Isoform 5b of tartrate-resistant acid phosphatase (TRACP-5b; interassay coefficient of variation ≤ 9.0%; Nittobo Medical Co. Ltd., Tokyo, Japan) was measured as a bone resorption marker (TRACP-5b demonstrates superior sensitivity and signal-to-noise ratio compared to serum cross-linked C-telopeptide of type I collagen (CTX) [13]). Serum 25-hydroxycholecalciferol (25(OH)D) levels were assessed via electrochemiluminescence using the Elecsys system (Roche Diagnostics, Basel, Switzerland).
Radiographs
Spinal radiographs were routinely obtained at baseline and subsequently at 6-month intervals following ROMO initiation [14]. Vertebral fractures with grades ≥ 1 were defined using the SQ method [10]. For patients exhibiting symptoms of incidental clinical, vertebral, or nonvertebral fractures, each attending investigator evaluated unscheduled radiographs.
Statistical analysis
Changes in BMD and bone turnover marker levels were evaluated based on the percentage change from baseline. The Mann–Whitney U test, chi-square test, and Fisher exact test were employed for statistical comparisons between the two groups. Changes in BMD and bone turnover marker levels from baseline to specified time points within each study group were assessed using the Wilcoxon signed-rank test. Multiple regression analysis, examining associations with RA-specific factors and increase in BMD at 12 months (incorporating the LS as a region abundant in trabecular bone and the FN as a region abundant in cortical bone), was conducted by incorporating variables known to affect BMD in RA patients based on previous reports (anti-citrullinated peptide antibody (ACPA) titer [15], clinical disease activity index (CDAI) [16], health assessment questionnaire disability index (HAQ-DI) [2], and biological disease-modifying antirheumatic drugs (bDMARDs) [17] or Janus kinase inhibitors (JAKi) [18] usage). The number of variables utilized in the multiple regression analysis was determined in accordance with a previously reported methodology, which defined the number of variables by dividing the number of cases by 15 [19].
All statistical analyses were performed using EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which provides a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) [20]. P values < 0.05 were considered indicative of statistical significance.
Propensity score matching
To align the clinical backgrounds that could potentially influence bone metabolism, we implemented 1:1 optimal propensity score matching without replacement. This matching process included variables such as age, body mass index, BMD (T-score) of LS, TH, and FN, as well as differences in prior osteoporosis treatment (naïve, switched from bisphosphonates, denosumab, or teriparatide) as previously described [21].
Ethical statement
This study was conducted following the ethical standards of the Declaration of Helsinki. It received approval from the institutional ethical review board of Osaka University Graduate School of Medicine (approval No. 18258) and each participating institute. Informed consent was obtained from the patients, and opt-out information was made available on the hospital’s homepage.
Results
Patient disposition and characteristics
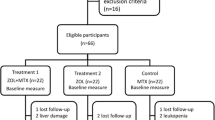
The detailed patient flow is presented in the CONSORT flow diagram (Fig. 1). Among 209 patients who initiated treatment with ROMO, 171 patients met the inclusion criteria. Patients were divided into two groups: the RA group (n = 59) and the non-RA group (n = 121). After propensity score matching, 41 patients from each group were selected.
Table 1 presents the clinical characteristics of the propensity score-matched patients at the time of ROMO induction. No significant differences were observed among the groups, except for corrected serum calcium levels. In the RA group, 80.5% of the patients were ACPA positive (mean titer; 206.2 U/ml), 53.7% of the patients were remission or low disease activity evaluated by CDAI (≤ 10), and 39.0% of the patients were HAQ remission (≤ 0.5). Additionally, 31.7% of patients were treated with bDMARDs or JAKi. The clinical characteristics of the non-matched patients at the time of ROMO induction are shown in Supplementary Table 1.
Bone turnover markers
Figure 2a and b show the percentage change in serum PINP and TRACP-5b levels, respectively. The non-RA group tended to exhibit a higher rate of increase in PINP compared to the RA group from 6 to 12 months. Conversely, the non-RA group tended to show a greater rate of decrease in TRACP-5b compared to the RA group from 6 to 12 months, indicating a larger anabolic window in the non-RA group. The percentage change in serum PINP and TRACP-5b levels for the non-matched patients is provided in Supplementary Table 2.
Percentage change of serum PINP level (a) and TRACP-5b level (b). PINP, N-terminal type I procollagen propeptide; TRACP-5b, isoform 5b of tartrate-resistant acid phosphatase; RA, rheumatoid arthritis. Bars indicate mean ± standard error. *P < 0.05, **P < 0.01, ***P < 0.001; change within each treatment group compared with baseline
Changes in BMD
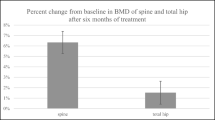
Regarding the percent change of BMD in the LS (Fig. 3a), the increase (mean ± standard error; P value compared with baseline) observed at 12 months was significantly higher in the non-RA group compared to the RA group (12.6 ± 1.0% vs. 9.1 ± 0.3%; P = 0.013), as well as in the TH (Fig. 3b) (4.8 ± 0.8% vs. 2.4 ± 0.6%; P = 0.025). In the FN, the non-RA group tended to show a higher BMD increase compared to the RA group at 12 months (Fig. 3c) (3.9 ± 1.0% vs. 2.3 ± 0.7%; P = 0.22). The percentage change in BMD for the non-matched patients is presented in Supplementary Table 3. In non-matched RA patients, treatment-naïve cases (n = 19) tended to show higher BMD increase compared to switched cases (n = 40) in the LS (10.5 ± 1.4% vs. 7.4 ± 0.8%; P = 0.078) and in the TH (3.7 ± 0.8% vs. 2.1 ± 0.8%; P = 0.21) at 12 months, although no significant difference was observed between the groups (Supplementary Table 4).
Percentage change of BMD in the lumbar spine (a), total hip (b), and femoral neck (c). BMD, bone mineral density; RA, rheumatoid arthritis. Bars indicate mean ± standard errors. #P < 0.05; difference between the two indicated groups. *P < 0.05, **P < 0.01, ***P < 0.001; change from baseline within each treatment group
Finally, we conducted a multiple regression analysis to investigate the association between RA-specific factors and an increase in LS or FN BMD at 12 months of non-matched RA patients (n = 59; Table 2). As a result, BMD increase in the LS was negatively associated with ACPA titer (OR = − 0.0052, 95% CI = − 0.0097– − 0.00071, P = 0.024) and positively associated with CDAI (OR = 0.162, 95% CI = 0.0281–0.297, P = 0.019). Conversely, BMD increase in the FN was negatively associated with HAQ-DI (OR = − 3.101, 95% CI = − 4.798– − 1.403, P = 0.00061).
Incidence of fragility fractures
In the RA group, one patient experienced a proximal humerus fracture due to a fall. In the non-RA group, one patient suffered a vertebral fracture from a fall, and one patient experienced a stress fracture of the rib.
Discussion
To the best of our knowledge, this study represents the first comparison of the effects of ROMO between RA and non-RA patients with postmenopausal osteoporosis. In RA patients, the anabolic window tended to be narrower, resulting in a smaller increase in BMD compared to non-RA patients.
Previous studies investigating the effects of ROMO in RA patients have primarily focused on comparisons between ROMO and DMAb. Mochizuki et al. demonstrated that ROMO was more effective in increasing LS BMD than DMAb in RA patients with severe osteoporosis [22]. Conversely, Kobayakawa et al. reported that in RA patients receiving oral glucocorticoids (GC), the effects of ROMO on LS BMD increase were comparable to those of DMAb [8]. Taken together, ROMO may be no less effective than DMAb in RA patients, although differences in the effects of ROMO between RA and non-RA patients remain unclear.
Concerning the effects of ROMO in RA patients, factors influencing sclerostin production from osteocytes warrant consideration. TNF-α, glucocorticoids, and mechanical unloading promote sclerostin production from osteocytes, while sex hormones inhibit it [4]. These factors are closely associated with postmenopausal RA, and indeed, serum sclerostin levels are elevated in patients with RA compared to healthy controls [5].
Additionally, TNF-α induces the production of another Wnt inhibiting factor, Dickkopf-related protein 1 (Dkk-1), from synoviocytes [23], and serum Dkk-1 levels are also up-regulated in RA patients compared to healthy controls [5]. Moreover, we have previously reported that IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2/ERK and SHP2/PI3K/Akt2 pathways, apart from Wnt pathway [24].
Consequently, it is conceivable that Wnt-related bone formation is down-regulated not only by sclerostin but also by Dkk-1. Furthermore, IL-6 may inhibit an alternative bone formation pathway apart from Wnt, potentially leading to decreased bone formation in response to ROMO.
Regarding bone resorption, ACPA binds to citrullinated vimentin expressed on the surface of osteoclasts, inducing the expression of IL-8, which leads to their differentiation in an autocrine manner [25]. Indeed, a high ACPA titer is associated with increased bone resorption and decreased BMD in RA patients [15]. Therefore, patients with a high ACPA titer may exhibit reduced inhibition of bone resorption and a smaller increase in BMD in response to ROMO, particularly in the LS where osteoclasts are abundant.
A previous study demonstrated that greater physical disability, as evaluated by the HAQ, was negatively associated with femoral BMD in female patients with RA [2]. In ovariectomized monkeys, an increase of FN BMD results from remodeling inhibition (due to RANKL inhibition), modeling-based bone formation (due to Wnt signaling modulation via sclerostin inhibition), and secondary mineralization [26]. Reduced mechanical loading induces the production of both RANKL and sclerostin by osteocytes, leading to the promotion of osteoclast differentiation and inhibition of bone formation [27]. Therefore, patients with high HAQ scores may experience reduced mechanical loading in the femur, potentially impairing the effects of ROMO due to increased RANKL and sclerostin production.
Conversely, high disease activity, as evaluated by CDAI, was positively associated with BMD increases by ROMO treatment in the LS, and a similar trend was observed in the FN. A previous study demonstrated that higher disease activity is associated with increased bone turnover in RA patients [5], and we have recently reported that baseline serum PINP levels were significantly associated with BMD increases by ROMO treatment in patients with postmenopausal osteoporosis [28]. Indeed, baseline PINP levels and CDAI tended to be correlated (odds ratio = 1.37, 95% CI = − 0.35–3.10, P = 0.12), and baseline PINP levels and the percent increase in BMD in the LS at 12 months showed a significant correlation (odds ratio = 5.77, 95% CI = 1.20–10.33, P = 0.014) in the non-matched RA group (n = 59) of this study.
This study has several limitations. Firstly, due to its retrospective, case-controlled design, there may be some selection bias in the baseline patient characteristics, which could have influenced the results. The RA patients included in this study had relatively long disease durations, with approximately half of them experiencing moderate to high disease activity and not taking oral glucocorticoids. Secondly, the statistical power of the results might be compromised due to the small number of patients included. Thirdly, we assessed serum TRACP-5b as a bone resorption marker, but serum CTX data were not available.
However, a notable strength of this study is that propensity score matching and multiple regression analysis were employed to mitigate variations in confounding factors related to postmenopausal osteoporosis between the RA and non-RA groups.
In conclusion, the efficacy of ROMO treatment may be attenuated in the RA group compared to the non-RA group, primarily due to a smaller anabolic window and subsequent BMD increases in the LS and TH. The effects of ROMO treatment may be influenced by RA-related factors, including ACPA titers and HAQ-DI.
Data Availability
The data reported in this paper is available from the corresponding author upon a reasonable request.
References
Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK (2000) Reduced bone mineral density in male rheumatoid arthritis patients: frequencies and associations with demographic and disease variables in ninety-four patients in the Oslo County Rheumatoid Arthritis Register. Arthritis Rheum 43:2776–2784
Haugeberg G, Uhlig T, Falch JA, Halse JI, Kvien TK (2000) Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheum 43:522–530
Tanaka S (2019) RANKL is a therapeutic target of bone destruction in rheumatoid arthritis. F1000Res 8:F1000 Faculty Rev-533
Delgado-Calle J, Sato AY, Bellido T (2017) Role and mechanism of action of sclerostin in bone. Bone 96:29–37
Terpos E, Fragiadaki K, Konsta M, Bratengeier C, Papatheodorou A, Sfikakis PP (2011) Early effects of IL-6 receptor inhibition on bone homeostasis: a pilot study in women with rheumatoid arthritis. Clin Exp Rheumatol 29:921–925
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, Langdahl BL, Reginster JY, Zanchetta JR, Wasserman SM, Katz L, Maddox J, Yang YC, Libanati C, Bone HG (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370:412–420
Gennari L, Rotatori S, Bianciardi S, Nuti R, Merlotti D (2016) Treatment needs and current options for postmenopausal osteoporosis. Expert Opin Pharmacother 17:1141–1152
Kobayakawa T, Miyazaki A, Kanayama Y, Hirano Y, Takahashi J, Suzuki T, Nakamura Y (2023) Comparable efficacy of denosumab and romosozumab in patients with rheumatoid arthritis receiving glucocorticoid administration. Mod Rheumatol 33:96–103
Orimo H, Nakamura T, Hosoi T, Iki M, Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, Soen S, Nishizawa Y, Hagino H, Fukunaga M, Fujiwara S (2012) Japanese 2011 guidelines for prevention and treatment of osteoporosis–executive summary. Arch Osteoporos 7:3–20
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Lewiecki EM, Binkley N, Morgan SL, Shuhart CR, Camargos BM, Carey JJ, Gordon CM, Jankowski LG, Lee JK, Leslie WD (2016) Best practices for dual-energy X-ray absorptiometry measurement and reporting: International Society for Clinical Densitometry Guidance. J Clin Densitom 19:127–140
Ebina K, Hirao M, Hashimoto J, Matsuoka H, Iwahashi T, Chijimatsu R, Etani Y, Okamura G, Miyama A, Yoshikawa H (2018) Impact of switching oral bisphosphonates to denosumab or daily teriparatide on the progression of radiographic joint destruction in patients with biologic-naive rheumatoid arthritis. Osteoporos Int 29:1627–1636
Nenonen A, Cheng S, Ivaska KK, Alatalo SL, Lehtimaki T, Schmidt-Gayk H, Uusi-Rasi K, Heinonen A, Kannus P, Sievanen H, Vuori I, Vaananen HK, Halleen JM (2005) Serum TRACP 5b is a useful marker for monitoring alendronate treatment: comparison with other markers of bone turnover. J Bone Miner Res 20:1804–1812
Ebina K, Etani Y, Tsuboi H, Nagayama Y, Kashii M, Miyama A, Kunugiza Y, Hirao M, Okamura G, Noguchi T, Takami K, Goshima A, Miura T, Fukuda Y, Kurihara T, Okada S, Nakata K (2022) Effects of prior osteoporosis treatment on the treatment response of romosozumab followed by denosumab in patients with postmenopausal osteoporosis. Osteoporos Int 33:1807–1813
Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, Jakobsson PJ, Baum W, Nimmerjahn F, Szarka E, Sarmay G, Krumbholz G, Neumann E, Toes R, Scherer HU, Catrina AI, Klareskog L, Jurdic P, Schett G (2012) Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest 122:1791–1802
Wysham KD, Shofer J, Lui G, Trupin L, Andrews JS, Black DM, Graf J, Shoback DM, Katz PP (2022) Low cumulative disease activity is associated with higher bone mineral density in a majority Latinx and Asian US rheumatoid arthritis cohort. Semin Arthritis Rheum 53:151972
Gulyas K, Horvath A, Vegh E, Pusztai A, Szentpetery A, Petho Z, Vancsa A, Bodnar N, Csomor P, Hamar A, Bodoki L, Bhattoa HP, Juhasz B, Nagy Z, Hodosi K, Karosi T, FitzGerald O, Szucs G, Szekanecz Z, Szamosi S, Szanto S (2020) Effects of 1-year anti-TNF-alpha therapies on bone mineral density and bone biomarkers in rheumatoid arthritis and ankylosing spondylitis. Clin Rheumatol 39:167–175
Hamar A, Szekanecz Z, Pusztai A, Czokolyova M, Vegh E, Petho Z, Bodnar N, Gulyas K, Horvath A, Soos B, Bodoki L, Bhattoa HP, Nagy G, Tajti G, Panyi G, Szekanecz E, Domjan A, Hodosi K, Szanto S, Szucs G, Szamosi S (2021) Effects of one-year tofacitinib therapy on bone metabolism in rheumatoid arthritis. Osteoporos Int 32:1621–1629
Kabata D, Ayumi S (2019) Statistical analysis of observational study. J Japan Soc Pain Clin 26:1–6
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Nagayama Y, Ebina K, Tsuboi H, Hirao M, Hashimoto J, Yoshikawa H, Okada S, Nakata K (2022) Low serum albumin concentration is associated with increased risk of osteoporosis in postmenopausal patients with rheumatoid arthritis. J Orthop Sci 27:1283–1290
Mochizuki T, Yano K, Ikari K, Hiroshima R, Okazaki K (2023) Comparison of romosozumab versus denosumab treatment on bone mineral density after 1 year in rheumatoid arthritis patients with severe osteoporosis: a randomized clinical pilot study. Mod Rheumatol 33:490–495
Yeremenko N, Zwerina K, Rigter G, Pots D, Fonseca JE, Zwerina J, Schett G, Baeten D (2015) Tumor necrosis factor and interleukin-6 differentially regulate Dkk-1 in the inflamed arthritic joint. Arthritis Rheumatol 67:2071–2075
Kaneshiro S, Ebina K, Shi K, Higuchi C, Hirao M, Okamoto M, Koizumi K, Morimoto T, Yoshikawa H, Hashimoto J (2014) IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J Bone Miner Metab 32:378–392
Jung YK, Kang YM, Han S (2019) Osteoclasts in the inflammatory arthritis: implications for pathologic osteolysis. Immune Netw 19:e2
Ominsky MS, Libanati C, Niu QT, Boyce RW, Kostenuik PJ, Wagman RB, Baron R, Dempster DW (2015) Sustained modeling-based bone formation during adulthood in cynomolgus monkeys may contribute to continuous BMD gains with denosumab. J Bone Miner Res 30:1280–1289
Komori T (2013) Functions of the osteocyte network in the regulation of bone mass. Cell Tissue Res 352:191–198
Kashii M, Kamatani T, Nagayama Y, Miyama A, Tsuboi H, Ebina K (2023) Baseline serum PINP level is associated with the increase in hip bone mineral density seen with romosozumab treatment in previously untreated women with osteoporosis. Osteoporos Int 34:563–572
Acknowledgements
The authors thank Yasunori Tsukamoto, Yasuji Kato, and Hideki Yoshikawa as well as all the medical staff for their excellent cooperation in the conduct of this study.
Funding
Open Access funding provided by Osaka University.
Author information
Authors and Affiliations
Contributions
Study design: KE and YN. Study conduct: KE, YN, MK, YE, and TN. Data collection: KE, YN, MK, HT, GO, AM, YE, TN, TM, YF, and TK. Data analysis: KE, YN, and MK. Data interpretation: KE, YN, MK, HT, YE, and TN. Drafting the manuscript: KE and YN. Supervise: MH, KN, and SO. Approving the final version of the manuscript: KE, YN, MK, HT, GO, AM, YE, TN, MH, TM, YF, TK, KN, and SO. KE takes responsibility for the integrity of the data analysis.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
KE is affiliated with the Department of Musculoskeletal Regenerative Medicine, Osaka University, Graduate School of Medicine, which is supported by Taisho. KE has received research grants from Asahi-Kasei, Astellas, Eisai, Ono, and Teijin Pharma. KE has received payments for lectures from Abbive, Amgen, Asahi-Kasei, Astellas, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, Ono, Pfizer, Taisho, Tanabe-Mitsubishi, and Teijin Pharma. YN has received payments for lectures from Abbvie, Amgen, Asahi-Kasei, Astellas, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, and Tanabe-Mitsubishi. MK has received payments for lectures from Amgen, Asahi-Kasei, Astellas, and Daiichi Sankyo. HT has received a research grant from Chugai and has received payments for lectures from Abbive, Amgen, Asahi-Kasei, Astellas, Ayumi, Chugai, Eisai, Mochida, Pfizer, and Tanabe-Mitsubishi. GO has received payments for lectures from Eisai. YE and MH have received a research grant from Eli Lilly. KN has received a research grant from Astellas and supervises the Department of Musculoskeletal Regenerative Medicine, Osaka University Graduate School of Medicine, which is supported by Taisho. AM, TN, TM, YF, TK, and SO declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ebina, K., Nagayama, Y., Kashii, M. et al. An investigation of the differential therapeutic effects of romosozumab on postmenopausal osteoporosis patients with or without rheumatoid arthritis complications: a case–control study. Osteoporos Int 35, 841–849 (2024). https://doi.org/10.1007/s00198-024-07019-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-024-07019-2