Abstract
Summary
In men and women with opportunistically identifiable vertebral fractures (VFs) on routine CT scans including the chest and/or abdomen, the risk of death is 51% higher than in those with no VF on the CT scan, and 325% higher than an age- and sex-matched general population cohort.
Purpose
There is little knowledge about the risk of death in patients with VFs present on routine radiological imaging. We evaluated the risk of death in men and women aged 50 years or older with opportunistically identifiable VFs on routine CT scans and not treated with osteoporosis medications.
Methods
Thoracic and lumbar VFs were identified through a blinded, two-step approach on CT scans performed as part of normal clinical care in a Danish hospital in 2010 or later. Subjects with VF were matched on age and sex against those with no VF (1:2-ratio) and a general population cohort (1:3-ratio), respectively, and followed for up to 7 years through the national Danish registers. Subjects treated with an osteoporosis medication in the year prior to baseline were excluded.
Results
Subjects with VF had a significantly higher risk of death during follow-up as compared to subjects with no VF on the CT scan (adjusted hazard ratio [HR] 1.51 [95% confidence interval 1.27–1.79; p < 0.001]) and even more so when compared to the general population cohort (HR 4.25 [3.53–5.12; p < 0.001]). In subjects with versus without VF on the CT scan, the risk was higher in those with moderate or severe VF, in those with no malignancy prior to baseline, and in those with a lower Charlson comorbidity index score.
Conclusion
Subjects with VF available for identification on routine CT scans face a substantially increased risk of death. Opportunistic identification and reporting of VF is important to identify these patients to allow intervention if indicated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With age, VFs are an increasingly frequent adverse health event (except a small decline in incidence rates in the oldest old in some studies) [1,2,3], and they constitute a substantial part of incident fractures [4, 5]. Patients with VF are at increased risk of additional fractures [6, 7], and overall experience an impaired quality of life in terms of physical health [8].
In addition, a link between VFs and death exists. In subjects with clinical or radiographic VF, an increased mortality has been demonstrated in a substantial number of studies as compared to subjects with no VF or to the general population [1, 9,10,11,12,13,14,15,16,17]. The relative risk of death is generally higher in the first years – or even months – after a clinical VF [1, 10, 11, 18], and in younger as compared to older subgroups [1, 11,12,13].
Vertebral fractures – of which approximately 1 in 3 or less are clinically diagnosed [19, 20] – are frequently present on routine radiological investigations, yet the majority of these VFs go unreported [21,22,23,24]. Contrary to the extensive body of literature on the consequences of VFs in general, the impact of such opportunistically identifiable VFs on clinical outcomes – in a context of competing morbidities and healthcare priorities – is only scarcely evaluated. For mortality, a single publication has shown an increased cumulative mortality in older patients with VFs on CT pulmonary angiograms. However, the study lacked detailed baseline information on the participants as well as adjusted assessments of the relative risk of death [24].
The opportunistic identification of a VF may signal an opportunity for intervention to improve patient outcomes. However, given the lack of evidence on the burden of mortality in this particular patient population, more detailed studies are needed to delineate the potential and guide the development of such interventions. The primary objective of this analysis is to evaluate the risk of death in men and women with VF as compared to those without VF available for identification on CT scans performed as part of routine clinical practice and not treated with osteoporosis medications. As a secondary objective, we evaluate the risk of death in subjects with VF available on such routine CT scans as compared to an age- and sex-matched general population sample. We shall refer to the second objective as the scaling analysis.
Methods
We established an observational cohort study to evaluate the consequences of VFs available for identification on radiological imaging performed as part of routine clinical care, in subjects not treated with osteoporosis medications (OMs). The setting and fracture outcomes of this study have been described previously [25]. In this paper, we report the mortality findings from this study, which was a pre-specified, secondary outcome. In brief, we reevaluated 2,000 CT scans performed as part of daily clinical care to identify subjects with thoracolumbar VF, and linked these data to the national Danish registers to assess the risk of death during up to seven years of follow-up. This report follows the STROBE statement [26].
This study is covered by the Danish Data Protection Agency approval for Region Zealand healthcare research (REG-101–2018). Furthermore, the Danish Patient Safety Authority (3–3013-2687/1) and Statistics Denmark (707480) approved the study. The need for formal patient consent was waived by the authorities under Sect. 46 of the Danish Health Act, and ethics committee approval is not required for this type of study.
Study population
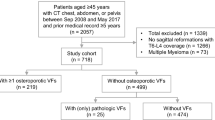
The first 2,000 men and women with a CT scan encompassing the chest and/or abdomen, performed at Holbæk Hospital (Denmark) from 1st January 2010 and onwards, were identified in the local radiology database and included in this study. Subjects needed to be 50 years or older at the time of the scan. The last included CT scan was performed in 2011. There were no requirements to the number of visible vertebrae per scan, as to mimic data available in clinical practice.
We retrieved the 2,000 scans – if subjects had multiple scans, we retrieved the first scan meeting the inclusion criteria – and re-evaluated them to identify cases with certain, potential or no VF. This triage was performed by one of the authors (CL). All cases with certain (n = 261) or potential (n = 207) VF, and a sample (5%) of scans with no VF (ntotal = 1,532), were then further evaluated by trained radiologists at an external radiology service (Clario, Princeton, NJ 08540, USA) to identify and grade prevalent VF from T1 to L5 (including L6 if available), according to the Genant Semiquantitative method [27]. Both steps in the re-evaluation process were blinded to clinical information. We did not include cervical VFs.
Information from this CT scan re-evaluation was linked with the national Danish registers. Using registry data, we excluded subjects with no or less than 1 year of data available prior to baseline (date of CT scan), those who migrated out of Denmark (and did not return) before baseline, or were treated with OM within the year before baseline. To assemble the analysis population, we then matched subjects with prevalent VF against those with no visible VF on age (at baseline, in 5-year age bands) and sex in a 1:2-ratio. Treatment with OM was defined as at least one filled prescription for a bisphosphonate, raloxifene, strontium ranelate, denosumab, or teriparatide, and/or at least one hospital administration of an intravenous bisphosphonate or denosumab.
We speculated that the CT population would constitute a selected, more diseased group as compared to the general population (referral bias). Therefore, to evaluate the risk of death in subjects with VF as compared to the general population, we matched subjects with VF on the CT scan on age group (in 5-year age bands according to birth year) and sex against a general population sample identified from the Danish registers. Pre-matching, subjects with VF on the CT scan were excluded following the procedure described above, while subjects from the general population sample were excluded if conflicting individual-level registry data were observed. General population sample subjects were excluded post-matching if < 1 year of registry data was available before baseline (date of CT scan of the matched case), the subject migrated out of Denmark (and did not return) prior to baseline, were treated with OM within the year before baseline, or if age < 50 years or dead at baseline. In the final step of assembling this scaling analysis population, matched comparator subjects were randomly selected in a 3:1-ratio.
For elaboration on sample size determination, please see Skjødt et al. (JBMR Plus, 2023) [25]. A post-hoc power calculation for the “primary” analysis reported in this paper estimated 80% power to detect a 15% increase in deaths (from 57% to 66.5%) based on a total N of 927 with 1 in 3 having a VF on the CT scan. The analysis had 85% power to detect an 18% increase in deaths, and 90% power to detect a 20% increase.
Outcomes
The primary outcome is the risk of all-cause death in subjects with VF on the CT scan as compared to subjects with no VF on the CT scan, assessed from baseline and up to 7 years of follow-up. The secondary outcome evaluates a similar endpoint in subjects with VF on the CT scan as compared to the general population sample (i.e. within the scaling analysis population). Finally, we examined the distribution of causes of death, using the primary cause of death for each deceased subject.
Date and cause of death were identified in the Danish Register of Causes of Death. Cause of death is listed in the register using the ICD-10 (International Classification of Diseases 10th revision) classification [28].
Covariate definition and sources
Date of birth, sex, migration, medical history, and use of pharmaceutical drugs (including hospital-administered drugs) were retrieved from the national Danish registers, using the Civil Registration System, the National Patient Register from 1994 onwards, and the National Prescription Register from 1995 onwards [29].
Baseline medical history is defined by the occurrence of at least one relevant diagnosis code prior to baseline or, for a small number of diseases, at least one filled prescription for certain medications. Medication use is defined by a filled prescription and/or hospital-administration of selected drugs. To calculate the Charlson Comorbidity Index (CCI) score we used the previously described updated weights [30]. For details and applied codes see Skjødt et al. (JBMR Plus, 2023), with codes specified in Supplemental Table 1 [25].
Statistical analyses
Baseline is the date of the CT scan. For the general population cohort, it is the date of the CT scan of the matched case. We present continuous baseline characteristics as median and interquartile range or mean and standard deviation, whereas counts and proportions are used for categorical characteristics.
For the risk of death, we report the number of deaths, the proportion of the cohorts who died, and the mortality rate; subjects contribute from baseline until censored. We used conditional Cox proportional hazards regression models to assess the risk of death in subjects with VF on the CT scan vs subjects with no VF on the CT scan (primary outcome), and vs the general population sample (secondary outcome). Subjects were censored at the time of death, emigration, initiation of OM, or at seven years follow-up, whichever occurred first.
Pre-specified adjusted Cox models were developed by backwards selection, using a p-value < 0.1 for inclusion in the final model. The covariates applied in the selection procedure were defined a priori for the primary endpoint of the overarching study – which was the risk of any subsequent fracture – and used here for consistencyFootnote 1 [25]. For the adjusted Cox models, statistical matching was lifted, and age and sex forced into the models. We examined exposure-confounder interactions; the confounders are those covariates included in the final model. In the analysis population, the interaction term with malignancies was borderline significant, while in the scaling analysis population it was statistically significant for age; therefore, we report exploratory subgroup analyses stratifying the outcome according to these variables. We used Schönfelds residuals to examine the proportional hazards assumption, and if statistically significant (p < 0.05) a time varying covariate was implemented in the adjusted model, using the logarithmic function of time. The proportional hazards assumption was not evaluated in the subgroup and sensitivity analyses.
Post-hoc subgroup and sensitivity analyses were performed for the primary outcome. Subgroup analyses include stratification by age group (50–59, 60–69, 70–79, and 80 +), sex, number (1, 2, 3, 4 +) of VFs, severity (mild, moderate, severe) of the worst VF, position (chest, lumbar, both) of VF, Charlson comorbidity index score (0, 1, 2, 3 +), and baseline presence of prior malignancies. These subgroups were evaluated by the implementation of interaction terms. Two sensitivity analyses were performed: 1) not censoring at the time of OM initiation; 2) only including comparators with the full thoracolumbar spine (T1-L5) visible on the CT scan. The latter sensitivity analysis was conducted to evaluate the potential impact of non-differential misclassification of the exposure, which could occur if a VF was present outside the CT field-of-view in the comparator subjects (who were deemed to have no VF on the CT scan).
There were no missing data for sex nor age. Loss to follow-up could occur if a subject migrated out of Denmark after baseline (at which point they would be censored from this study), yet this occurred in less than five subjects across the cohorts in both the analysis and the scaling analysis population, respectively.
We used Stata version 16 and 17 (StataCorp, College Station, Texas 77845, USA) to perform the analyses.
Results
Participants
Analysis population
Based on the re-evaluation of the CT scans, 423 (21.2%) of the 2,000 subjects had one or more VF on the CT scan. In the assembly of the analysis population (Table 1, panel a), 91 subjects (22%) were excluded from this VF group, while 61 (4%) were excluded from the no VF group. After matching, the VF on CT scan cohort consisted of 321 subjects while the no VF on CT scan cohort consisted of 606 subjects.
Mean age was 73 years in both the VF and no VF cohorts, while approximately 53–54% of the cohorts were male (Table 2). Baseline characteristics were largely similar across the cohorts, although a larger proportion of the VF cohort had a medical history of any major osteoporotic fracture in the year prior to baseline (9.0% vs 2.3%). Baseline characteristics stratified according to sex are available in the appendix, Table 6.
Scaling analysis population
For the scaling analysis population (Table 1, panel b), the VF group underwent an exclusion procedure similar to what is described above. For the general population sample, 20,000 subjects were identified in the registries as planned. Seventeen (0.1%) of these were excluded due to conflicting registry data. Following matching, post-matching exclusion from the general population sample (n = 805 [4.0%]), and random selection of matched pairs, the VF cohort was composed of 332 subjects, while the general population cohort consisted of 996 subjects.
Across the cohorts, mean age was 73–74 years and 54.5% were male (Table 2). The median CCI-score was higher in the VF cohort as compared to the general population cohort (2 vs 0). Larger proportions of the VF cohort had a history any major osteoporotic fracture (9.3% vs 1.0% in the year prior to baseline), type 1 diabetes mellitus (5.4% vs 2.2%), type 2 diabetes mellitus (12.0% vs 5.9%), and any malignancies (39.8% vs 12.4%). Baseline characteristics stratified according to sex are available in the appendix, Table 7.
Risk of death in subjects with VF vs without VF on the CT scan (analysis population)
In the VF cohort, 222 (69.2%) subjects died during a total of 804 years of follow-up, as compared to 345 (56.9%) subjects in the no VF cohort during 2,231 years of follow-up (Table 3). This translated into mortality rates of 276 (95% CI 242–315) and 155 (139–172) per 1,000 subject-years, respectively. Sex-specific mortality rates are listed in the appendix, Table 8. Overall, 13 (4%) and 27 (4%) subjects in the VF and no VF cohorts sustained at least one fracture (any fracture, except face, skull, and digits) between baseline and time of death, whereas 18 (6%) and 38 (6%) subjects sustained a fracture and did not die during follow-up. Censoring due to initiation of osteoporosis medication occurred in 39 (12%) of the subjects in the VF cohort, as compared to 43 (7%) in the no VF cohort.
The crude mortality hazard ratio (HR) was 1.59 (95% CI 1.34–1.89; p < 0.001). For the adjusted Cox model – with covariates identified by backwards selection – we incorporated a time-varying covariate (age) because the proportional hazards assumption was not met. The subsequent model yielded a mortality HR of 1.51 (1.27–1.79; p < 0.001), adjusted for sex, age, baseline presence of systemic lupus erythematosus, malignancies, type 1 diabetes mellitus, type 2 diabetes mellitus, ever use of antidepressants, and ever use of glucocorticoids.
These findings are reflected in the Kaplan–Meier failure function (Fig. 1, panel a). The difference in mortality rates between the VF and no VF cohorts is clearly visible as a steeper increase in the former group in the first 500 to 1,000 days from baseline, after which the curves become almost parallel, indicating much more similar mortality between the two groups with time.
This figure shows the Kaplan–Meier failure functions for the cumulative mortality in the analysis population and the scaling analysis population, respectively. Also shown is the adjusted hazard ratio (HR) for death in the respective population. Panel a: Subjects with VF on the CT scan vs subjects with no VF on the CT scan (analysis population). Hazard ratio adjusted for sex, age (tvc), baseline presence of systemic lupus erythematosus, malignancies, type 1 diabetes mellitus, type 2 diabetes mellitus, ever use of antidepressants, and ever use of glucocorticoids. Matching lifted to perform this analysis. Panel b: Subjects with VF on the CT scan vs a general population comparator cohort (scaling analysis population). Hazard ratio adjusted for sex, age (tvc), baseline presence of systemic lupus erythematosus, Bechterew’s disease (ankylosing spondylitis), malignancies, type 1 diabetes mellitus, type 2 diabetes mellitus, and ever use of proton pump inhibitors. Matching lifted to perform this analysis. CI, confidence interval; CT, computed tomography; general pop., general population; GP, general population; HR, hazard ratio; tvc, time-varying covariate; VF, vertebral fracture
Subgroup analyses for the primary outcome (Table 4) reflects a higher risk of death in female vs male subjects, and in those aged 60–69 years while lowest in the oldest subgroup (80 + years). Similarly, mortality was higher in subjects with moderate or severe VF at baseline, in those with both chest and lumbar VF as compared to either location alone, in those with a CCI score of 0 or 1 as compared to 2 or 3 + , and in those with no prior malignancies at baseline. While the p-values for interaction were statistically significant for the subgroup analysis by number of VF at baseline, the findings were not clinically meaningful (showing a higher risk in those with 2 or 4 + VF as compared to those with 1 or 3 VF). Sensitivity analyses found no clinically relevant impact of not censoring upon AOM initiation, while only including those comparator subjects with the full thoracolumbar (T1-L5) spine available on the CT yielded a reduction in the adjusted HR point estimate (1.32; 0.99–1.75).
Risk of death in subjects with VF vs the general population (scaling analysis population)
In the scaling analysis population, 231 (69.6%) and 287 (28.8%) subjects in the VF and general population cohorts died during 825 and 5,529 years of follow-up, respectively (Table 3). This yielded mortality rates of 280 (246–319) and 52 (46–58) deaths per 1,000 subject-years. Again, Table 8 shows the sex-specific mortality rates.
The crude HR was 6.54 (5.38–7.93; p < 0.001). In the analysis of the proportional hazards assumption for both the unadjusted and adjusted Cox models, Schönfelds residuals were highly statistically significant (p < 0.001). Therefore, we again introduced a time-varying covariate (age) in the adjusted model, which then yielded a HR of 4.25 (95% CI 3.53–5.12; p < 0.001), when adjusting for sex, age, baseline presence of systemic lupus erythematosus, Bechterew’s disease (ankylosing spondylitis), malignancies, type 1 diabetes mellitus, type 2 diabetes mellitus, and ever use of proton pump inhibitors. Because of significant interaction between exposure and age, we assessed mortality in subgroups of age, and found a markedly higher risk of death in the younger subgroups: adjusted HR 24.60 (7.11–85.16) if 50–59 years, 7.82 (5.09–12.01) if 60–69 years, 4.40 (3.27–5.91) if 70–79 years, and 2.76 (2.06–3.69) if 80 + years (p for interaction < 0.001).
The Kaplan–Meier failure function (Fig. 1, panel b) is consistent with the overall findings, showing a stable mortality rate throughout follow-up in the general population cohort, while in the VF cohort a steep increase is observed shortly after baseline, and with the curves continuously diverging throughout the follow-up period.
Cause of death
Neoplasms, diseases of the circulatory system and diseases of the respiratory system were the most frequently observed causes of death (Table 5). We observed only minor differences between the VF and no VF cohorts (analysis population) in the distribution of causes of death, with neoplasms being slightly more common in the no VF cohort (48.4% vs 43.2%).
Within the scaling analysis population, a substantially larger proportion of VF subjects had neoplasms listed as the primary cause of death (43.3% vs 23.3%), while the general population subjects more frequently died from mental and behavioural disorders (7.7% vs 2.2%) and diseases of the circulatory system (34.8% vs 14.7%).
Discussion
This study found a substantially increased risk of death in subjects with opportunistically identifiable VF on CT scans obtained during routine practice. This was observed both in comparison to subjects with no VF on routine CT scans, and – even more pronounced – when compared to a general population sample. The difference in mortality rates between the cohorts was most notable early after baseline, and the mortality became more similar over time. Critically, the impact of VF on mortality was strongest in subgroups with little or no comorbid conditions, and in those with no prior malignancies; it was also stronger in women than in men, in those aged 60–79 years, and if the VF was moderate or severe. We found no major differences between the VF and no VF cohorts in the distribution of cause of death, while subjects in the VF cohort died substantially more frequently from neoplasms and less frequently from mental and behavioural disorders as well as diseases of the circulatory system as compared to the general population sample.
The association between VFs and an increased risk of death has been shown previously in terms of both clinical VF as well as radiographic (prevalent) VF [1, 9,10,11,12,13,14,15,16,17], although it must be noted that a few studies generally failed to replicate this finding [31, 32]. The first of these two studies, however, did show that the subgroup of women with two or more radiographic VFs had a statistically significant 56% increased risk of death as compared to those with no VFs; this was not shown in men [31]. The latter study showed that women with incident radiographic VFs sustained over an average of 3.7 years had a 32% increased mortality in age-adjusted analyses, but this finding disappeared in the fully adjusted analyses (relative hazard 1.06 [95% confidence interval 0.88–1.28]) [32]. The findings from our study extend the VF-mortality association to also include patient populations with VFs identified opportunistically on CT scans. This has only been assessed in one prior study, which was confined to a fairly narrow clinical context: Jones et al. (Geriatrics, 2020) demonstrated a crude cumulative mortality of 68.5% in older subjects (75 + years) with VF on CT pulmonary angiograms vs 45.8% in subjects without such VFs (p = 0.0063) during up to 3.5–4.5 years of follow-up [24]. This is comparable to our mortality estimates of 69.2% and 56.9% in those with vs without VFs on routine CT scans. As compared to the previous study, the novelty of our analyses is observed in the detailed survival analysis risk estimates, and the comparison of subjects with VF on CT scans to those with no VF on such CT scans (a broader source population, thus extending the generalizability of our findings) and to a general population sample.
Longitudinal studies have noted an increasing risk of death by number and severity of VF (at least numerically) in patients with radiographic VF identified purposively at baseline [14, 17, 33]. While this has not previously been evaluated in subjects with opportunistically identifiable VF, we showed a higher risk of death in those with moderate or severe VF, whereas in subjects with mild VF the increased HR did not reach statistical significance. While the mortality risk point estimates generally increased with the number of VFs at baseline, we found the highest risk in those with 2 VFs. The reasons for this are not clear, yet it may merely reflect a higher base risk in this subgroup, or the presence of VF outside the CT field-of-view leading to an erroneously low VF count among subjects in this subgroup. Another notable finding from the subgroup analyses was the higher impact of prevalent VF in those with a CCI-score of 0 or 1 as compared to a CCI-score of 2 or 3 + . It could be speculated that there is a saturation of morbidity, so that the baseline risk of death in those with a CCI-score of at least 2 is already so high that also having a VF does not significantly add to that risk. An analogous inference could be made about subjects with vs without prior malignancies; subjects with prior diagnosis code(s) for any malignancy may be at such increased risk of death that also having a VF contributes less to mortality risk than in subjects with no prior malignancies. An alternative explanation could arise if subjects with advanced disease – leading to a higher risk of death – and prevalent VF are less likely to undergo CT scans as part of their routine care for other conditions than similar subjects without VF (i.e. referral bias based on severity of osteoporosis). If present, this would mean that the observed risk estimates in VF subjects within the high CCI- and any malignancy-strata are underestimated. Finally, we observed a reduction in the risk estimate when excluding comparators from the analysis population if the full T1 to L5 spine was not available on the CT scan. This may reflect that subjects undergoing a CT scan including the full thoracolumbar spine overall constitute a subpopulation with a particularly high risk of death, hence the effect on mortality of prevalent VF in this group becomes less impactful. The wide confidence intervals of this sensitivity analysis should be noted.
In terms of prevalent comorbidities and risk of death, a recent study identified clusters of comorbidities in Danish fracture patients, and showed how the excess 1-year mortality following VF was highest for those patients in the malignant comorbidity cluster, followed by the hepatic and/or inflammatory cluster (men only), and then the cardiovascular or diabetic cluster (depending on sex) [34]. If these comorbidity clusters translates into distinct causes of death was not examined, but several other studies have evaluated the cause of death specifically in VF subjects. In a study population of all ages, Choi et al. (Spine, 2020) found that larger proportions of subjects died in the VF cohort – as compared to a matched general population control group without VF – due to infections, neoplasms, neurologic diseases, circulatory diseases, respiratory diseases, digestive diseases, muscular diseases, and trauma. While statistically significant, the absolute differences between the cohorts were negligible (maximum of 0.7%-points of the respective cohorts) [12]. In the Study of Osteoporotic Fractures, the risks of death due to cancer and pulmonary diseases, respectively, were higher in those with vs those without VF [14]. In comparison, Tran et al. (JCEM, 2018) listed the primary cause of death in subjects with VF in Denmark, and found that the major sources of mortality were diseases of the circulatory or respiratory systems, together with neoplasms [15]. We found similar causes of death to be predominant across the cohorts. However, while we showed an overall increased risk of death in those with vs those without VF on CT scans, there were no major discernible differences between the cohorts in the distribution of the primary cause of death. It may be that presence of a VF in this setting is a marker of an overall reduced resilience to adverse health events. In the scaling analysis population, a larger proportion of deaths in subjects with VF were due to neoplasms, while smaller proportions died from circulatory diseases as well as mental and behavioural disorders.
Following our study, at least two major topics remain unresolved in the discussion of VF and mortality. First, the amount of excess mortality due to the VF itself as compared to the amount due to comorbidities. While Kanis et al. (Osteoporos Int, 2004) suggested that 28% of the excess mortality after a clinical VF was linked to the VF (the rest being due to the burden of comorbidities) [3], more research is needed to explore the underlying mechanisms and the specific contributions of each. Second, interventions to modify this increased risk of death need to be explored. To this end, one study found that in subjects with any fracture, subsequent fractures contributed > 10% to population attributable risk of death [11]. Similarly, substantially increased risks of death have been shown in subjects with any subsequent fracture(s) vs those with only one fracture [4], while a study from Taiwan reported significantly increased hazard ratios for death during a 2-year follow-up in subjects with an index VF and subsequent hip (HR 1.99 [95% CI 1.87–2.13]), vertebral (1.10 [1.00–1.20]), or upper humerus (1.64 [1.39–1.93]) fracture as compared to those with only the index VF [35]. Thus, it would be logical to infer that avoiding subsequent fractures by treating the osteoporosis would reduce mortality risk. In our study, 31 of 321 and 65 of 606 subjects in the VF and no VF cohorts, respectively, sustained at least one fracture after baseline [25]; in both cohorts, 42% of these patients died during the remainder of the follow-up. While our study was not powered for such analyses, the previous findings indicate, as noted, that secondary fracture prevention may be important in reducing mortality. Accordingly, implementation of a fracture liaison service (FLS) has been associated with reductions in mortality (adjusted HR 0.84 [95% CI 0.73–0.96]) in patients belonging to a composite index fracture group (hip/pelvis/proximal humerus or tibia/vertebral/multiple rib/distal femur); a significant reduction in mortality was not observed in patients with other fractures (similarly pooled in one composite group) [36]. However, a meta-analysis of clinical trials of pharmaceutical anti-fracture treatments for osteoporosis showed no statistically significant effect of these treatments on mortality [37]. Similarly, Levy and colleagues (2012) found no significant effect of vertebro- or kyphoplasty nor osteoporosis treatment (alone or in combination, as compared to no treatment) on mortality in patients with VF [38], although small numbers and residual confounding may have influenced their findings. Together, this highlights the need for additional studies on the cause and potential mitigation of the excess mortality following VF. An important aspect is if VF subjects survive long enough to benefit from OM treatment. From the risk table in Fig. 1a, 159/321 (50%) of VF subjects remain at risk (i.e. alive, residing in Denmark, and not treated with OM) at 500 days after baseline. It seems reasonable to assume that most of these subjects would have benefitted from timely VF identification and subsequent fracture risk assessment.
Strengths of this study include the minimal loss to follow-up and the possibility for long-term follow-up. Furthermore, the use of routine CT scans in an observational study design with registry-based follow-up makes the results directly applicable to clinical practice; however, as this was a single-center study using only CT scans to identify baseline VF, generalizability to other countries and imaging modalities may be reduced. Another limitation is that the referral for the CT scan was not available in this study, precluding subgroup analyses according to CT scan indication (e.g. trauma, cancer, etc.). Other limitations include a risk of residual confounding in the adjusted risk estimates, as important mortality risk factors – e.g. smoking – are not available in the Danish registers. We would, however, expect that most of the risk imparted by such missing covariates is reflected in the available medical history of the subjects in this trial. Similarly, as the covariates applied in the backwards selection procedure for the adjusted models were a priori chosen to align with the primary outcome of the overarching study (fractures) [25], relevant confounders may have been omitted from the adjusted analyses. Important covariates – e.g. cancer and diabetes – were, however, included in the model development. It is important to note that this study does not aim to delineate the pathophysiological paths by which the presence of a VF may influence survival prospects. This would have necessitated a random population screening study with invitation to VF imaging and subsequent observational follow-up without intervention, something that would have been ethically problematic. As can be seen clearly in the baseline tables, the patients who undergo CT scans are a highly selected population, and the presence of some comorbid conditions will increase the probability of a CT exam while other comorbidities may have the opposite influence. However, this reflects clinical decision-making, and only patients who – based on these decisions – undergo a CT scan (or other imaging) present an opportunity for opportunistic VF identification. Therefore, focusing on this group is essential to gain an understanding of the rationale for implementing screening for VF.
In conclusion, this study demonstrated that patients with VF available for identification on CT scans performed as part of routine clinical care and not treated for osteoporosis, are at increased risk of death as compared to those without visible VF on such scans, and even more so when compared to a general population sample. This highlights the importance of identification and reporting of opportunistically identifiable VF, and the need for tailored management strategies to overcome this increased mortality.
Data availability
MKS and CDS had full access to individual-level data of all subjects in this study and performed the data analysis. Under Danish law sharing of individual-level data hosted with Statistics Denmark is not possible.
Notes
Baseline covariates applied in the selection procedure: History of any prior fracture (except face, skull, and fingers), osteoporosis, Paget’s disease, any malignancy, benign tumours, hyperthyroidism, primary hyperparathyroidism, Cushing’s syndrome, hypogonadism, acromegaly, Addison’s disease, monogenetic osteoporosis, anorexia nervosa, bulimia, malabsorptive diseases, inflammatory bowel disease, pernicious anaemia, Bechterew’s disease (ankylosing spondylitis), mastocytosis, systemic lupus erythematosus, juvenile arthritis, rheumatoid arthritis, myelomatosis, type 1 diabetes mellitus, type 2 diabetes mellitus, and any prior use of sodium glucose cotransporter-2 (SGLT-2) inhibitors, thiazolidinediones, glucocorticoids, anticoagulants, antidepressants, cancer agents, antiepileptics, GnRH agonists, and proton pump inhibitors.
References
Lee Y-K, Jang S, Jang S et al (2012) Mortality after vertebral fracture in korea: analysis of the national claim registry. Osteoporos Int 23:1859–1865. https://doi.org/10.1007/s00198-011-1833-5
Bouza C, López T, Palma M, Amate JM (2007) Hospitalised osteoporotic vertebral fractures in Spain: analysis of the national hospital discharge registry. Osteoporos Int 18:649–657. https://doi.org/10.1007/s00198-006-0292-x
Kanis JA, Oden A, Johnell O et al (2004) Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 15:108–112
Lee S-B, Park Y, Kim D-W et al (2021) Association between mortality risk and the number, location, and sequence of subsequent fractures in the elderly. Osteoporos Int 32:233–241. https://doi.org/10.1007/s00198-020-05602-x
Amin S, Achenbach SJ, Atkinson EJ et al (2014) Trends in fracture incidence: a population-based study over 20 years. J Bone Miner Res 29:581–589. https://doi.org/10.1002/jbmr.2072
Gehlbach S, Saag KG, Adachi JD et al (2012) Previous fractures at multiple sites increase the risk for subsequent fractures: the global longitudinal study of osteoporosis in women. J Bone Miner Res 27:645–653. https://doi.org/10.1002/jbmr.1476
Schousboe JT, Vo T, Taylor BC et al (2016) Prediction of incident major osteoporotic and hip fractures by trabecular bone score (TBS) and prevalent radiographic vertebral fracture in older men. J Bone Miner Res 31:690–697. https://doi.org/10.1002/jbmr.2713
Al-Sari UA, Tobias J, Clark E (2016) Health-related quality of life in older people with osteoporotic vertebral fractures: a systematic review and meta-analysis. Osteoporos Int 27:2891–2900. https://doi.org/10.1007/s00198-016-3648-x
Tran T, Bliuc D, van Geel T et al (2017) Population-wide impact of non-hip non-vertebral fractures on mortality. J Bone Miner Res 32:1802–1810. https://doi.org/10.1002/jbmr.3118
Melton III LJ, Atkinson EJ, St. Sauver JL et al (2014) Predictors of excess mortality following fracture: a population-based cohort study. J Bone Miner Res 29:1681–1690. https://doi.org/10.1002/jbmr.2193
Bliuc D, Nguyen ND, Milch VE et al (2009) Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 301:513–521. https://doi.org/10.1001/jama.2009.50
Choi HG, Lee JK, Sim S, Kim M (2020) Mortality and cause of death in patients with vertebral fractures: a longitudinal follow-up study using a national sample cohort. Spine (Phila Pa 1976) 45:E280–E287
Lau E, Ong K, Kurtz S et al (2008) Mortality following the diagnosis of a vertebral compression fracture in the medicare population. J Bone Jt Surg 90:1479–1486. https://doi.org/10.2106/JBJS.G.00675
Kado DM, Browner WS, Palermo L et al (1999) Vertebral fractures and mortality in older women: a prospective study. Arch Intern Med 159:1215–1220
Tran T, Bliuc D, Hansen L et al (2018) Persistence of excess mortality following individual nonhip fractures: a relative survival analysis. J Clin Endocrinol Metab 103:3205–3214. https://doi.org/10.1210/jc.2017-02656
Pongchaiyakul C, Nguyen ND, Jones G et al (2005) asymptomatic vertebral deformity as a major risk factor for subsequent fractures and mortality: a long-term prospective study. J Bone Miner Res 20:1349–1355. https://doi.org/10.1359/JBMR.050317
Ikeda Y, Sudo A, Yamada T, Uchida A (2010) Mortality after vertebral fractures in a Japanese population. J Orthop Surg 18:148–152. https://doi.org/10.1177/230949901001800204
Johnell O, Kanis JA, Odén A et al (2004) Mortality after osteoporotic fractures. Osteoporos Int 15:38–42. https://doi.org/10.1007/s00198-003-1490-4
Fink HA, Milavetz DL, Palermo L et al (2005) What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res 20:1216–1222
Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ III (1992) Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 7:221–227
Williams AL, Al-Busaidi A, Sparrow PJ et al (2009) Under-reporting of osteoporotic vertebral fractures on computed tomography. Eur J Radiol 69:179–183. https://doi.org/10.1016/j.ejrad.2007.08.028
Bazzocchi A, Spinnato P, Albisinni U et al (2012) A careful evaluation of scout CT lateral radiograph may prevent unreported vertebral fractures. Eur J Radiol 81:2353–2357. https://doi.org/10.1016/j.ejrad.2011.08.015
Li Y, Yan L, Cai S et al (2018) The prevalence and under-diagnosis of vertebral fractures on chest radiograph. BMC Musculoskelet Disord 19:235. https://doi.org/10.1186/s12891-018-2171-y
Jones L, Singh S, Edwards C et al (2020) Prevalence of vertebral fractures in CTPA’s in adults aged 75 and older and their association with subsequent fractures and mortality. Geriatrics 5:56. https://doi.org/10.3390/GERIATRICS5030056
Skjødt MK, Nicolaes J, Smith CD et al (2023) Fracture risk in men and women with vertebral fractures identified opportunistically on routine computed tomography scans and not treated for osteoporosis: an observational cohort study. JBMR Plus 7:e10736. https://doi.org/10.1002/jbm4.10736
Vandenbroucke JP, von Elm E, Altman DG et al (2007) Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 4:1628–1654. https://doi.org/10.1371/journal.pmed.0040297
Genant HK, Wu CY, Van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Helweg-Larsen K (2011) The danish register of causes of death. Scand J Public Health 39:26–29. https://doi.org/10.1177/1403494811399958
Schmidt M, Schmidt SAJ, Adelborg K et al (2019) The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 11:563–591. https://doi.org/10.2147/CLEP.S179083
Quan H, Li B, Couris CM et al (2011) Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173:676–682. https://doi.org/10.1093/aje/kwq433
Trone DW, Kritz-Silverstein D, von Mühlen DG et al (2007) Is radiographic vertebral fracture a risk factor for mortality? Am J Epidemiol 166:1191–1197. https://doi.org/10.1093/aje/kwm206
Kado DM, Duong T, Stone KL et al (2003) Incident vertebral fractures and mortality in older women: a prospective study. Osteoporos Int 14:589–594
Hasserius R, Karlsson MK, Nilsson BE et al (2003) Prevalent vertebral deformities predict increased mortality and increased fracture rate in both men and women: a 10-year population-based study of 598 individuals from the Swedish cohort in the European Vertebral Osteoporosis Study. Osteoporos Int 14:61–68. https://doi.org/10.1007/s00198-002-1316-9
Tran T, Bliuc D, Ho-Le T et al (2022) Association of multimorbidity and excess mortality after fractures among danish adults. JAMA Netw Open 5:e2235856. https://doi.org/10.1001/jamanetworkopen.2022.35856
Chen J-L, Tai T-W, Chou C-Y et al (2022) Incidence of different types of subsequent fractures and related mortality in Taiwan. Arch Osteoporos 17:55. https://doi.org/10.1007/s11657-022-01098-6
Vranken L, de Bruin IJA, Driessen AHM et al (2022) decreased mortality and subsequent fracture risk in patients with a major and hip fracture after the introduction of a fracture liaison service: a 3-year follow-up survey. J Bone Miner Res 37:2025–2032. https://doi.org/10.1002/jbmr.4674
Cummings SR, Lui L-Y, Eastell R, Allen IE (2019) Association between drug treatments for patients with osteoporosis and overall mortality rates: a meta-analysis. JAMA Intern Med 179:1491–1500. https://doi.org/10.1001/jamainternmed.2019.2779
Levy H, Seydafkan S, Rice JD et al (2012) Comparative efficacy of vertebroplasty, kyphoplasty, and medical therapy for vertebral fractures on survival and prevention of recurrent fractures. Endocr Pract 18:499–507. https://doi.org/10.4158/EP11349.OR
Acknowledgements
We are indebted to Sören Möller (OPEN—Open Patient data Explorative Network, Department of Clinical Research, University of Southern Denmark and Odense University Hospital, Odense, Denmark) for statistical support, and Preben Trunshøj (independent), who performed the acquisition and pseudonymization of the CT scans from the Holbæk Hospital radiology database.
Funding
Open access funding provided by Zealand Region This is an investigator initiated study funded by a grant from the Region Zealand Health Scientific Research Foundation and by a collaborator grant from UCB Pharma/Amgen Inc. The lead author was supported by a PhD scholarship from the Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark. CL and JN are UCB Pharma employees, and the manuscript was shared with UCB Pharma/Amgen Inc. for courtesy review prior to submission (from this review, comments on the manuscript were received from one of the authors [CL], who was also part of the courtesy review panel; beyond this, no comments were received). The other funders were not involved in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All authors had full access to the study results, and assumes responsibility for the decision to submit for publication.
Conflicts of interest
MKS and BA have received support for this study from UCB/Amgen and Region Zealand Health Scientific Research Foundation (research grants with funds paid to the institution); MKS has received support from the University of Southern Denmark (PhD scholarship), UCB (educational grant) outside the submitted work, UCB Nordic (personal speaker fees), is a board member of the Danish Bone Society, and a member of working groups in the Danish Bone Society and the European Calcified Tissue Society; JN and CL are employees of UCB Pharma with stock ownership, and JN is involved in a patent (11341639) and has received support for travel from UCB Pharma; CDS and KRO have no conflicts to report; CC has received personal fees from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda, and UCB; BA has received personal speakers fees/consulting fees from UCB, Amgen, Kyowa-Kirin, and Pharmacosmos, and institutional research grants (with funds paid to the institution) from Novartis, Kyowa-Kirin, and Pharmacosmos, and is the past-president of the European Calcified Tissue Society.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Skjødt, M.K., Nicolaes, J., Smith, C.D. et al. Opportunistically identifiable vertebral fractures on routine radiological imaging predict mortality: observational cohort study. Osteoporos Int 35, 691–703 (2024). https://doi.org/10.1007/s00198-024-07017-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-024-07017-4