Abstract
Summary
Most studies investigating the association between physical activity and osteoporosis prevention only focused on specific types of physical activity. This study’s evidence regarding the combined effects or interaction of sleep duration and physical activity. The findings emphasize the role of sleep duration and physical activity in association with osteoporosis.
Purpose
The associations between physical activity, sleep duration, and prevalent osteoporosis in Taiwanese adults were studied in this cross-sectional study.
Methods
The Taiwan Biobank enrolled a community-based cohort of ~ 120,000 volunteers (as of April 30, 2020) between 30 and 76 years of age with no history of cancer. Amongst, bone mineral density (BMD) measures by dual-energy X-ray absorptiometry (DXA) were available in 22,402 participants. After excluding individuals who had no complete data of BMI (n = 23), MET score (n = 207), T-score (n = 8,826), and sleep duration (n = 16), 13,330 subjects were included as the primary cohort. Univariate and multivariable regression analyses were performed to determine the associations between the presence of osteoporosis, physical activity level, sleep duration, and other variables.
Results
The results showed that after adjustment, subjects with physical activity < 20 METs/week and ≥ 20 METs/week (aOR = 1.017 and 0.767, respectively) were associated with risk of osteoporosis than those with zero MET. The odds of osteoporosis were not significantly lower in subjects who slept for ≥ 8 h/day (aOR = 0.934,p=0.266). In addition, compared to short sleepers with no physical activity, adults with increased physical activity ≥ 20 METs/week and sleep ≥ 8 h/day had a significantly lowest likelihood of osteoporosis (aOR = 0.702). Those with medium physical activity (< 20 METs/week) plus average sleep duration (6.5–8 h/day) did not have significant higher odds of osteoporosis (aOR = 1.129,p=0.151).
Conclusion
The findings emphasize the joint role of sleep duration and physical activity in association with osteoporosis. Adults with high physical activity plus high sleep hours have the highest BMD and lowest risk of osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a metabolic bone disease characterized by low bone mass, microarchitectural deterioration, and an increased risk of fragility fractures [1]. Bone mass and bone tissue is in a dynamic balance between the effect of bone formation and bone resorption [2]. When the balance is disrupted and the rate of bone resorption exceeds bone formation by any means, the bone mass is lost gradually [3]. According to the diagnostic criteria provided by World Health Organization, women with bone density levels more than 2.5 standard deviations below the young adult reference mean are considered to have osteoporosis [4, 5]. Risk factors for osteoporosis and osteoporotic fractures include non-modifiable factors such as older age, female gender, White ethnic background, family history of osteoporosis [6,7,8,9], and modifiable factors such as inadequate nutritional absorption, weight loss, cigarette smoking, alcohol consumption, and air pollution [10,11,12,13,14,15]. A data brief released by Center for Disease Control and Prevention indicated that the prevalence of osteoporosis in the United States at either femur neck or lumbar spine was 12.6% for adults aged more than 50 years old [16]. And a meta-analysis reported that the global osteoporosis prevalence was 18.3% [17]. Thus, it is necessary to completely understand all aspects of osteoporosis for the public’s health.
As part of the modifiable risk factors, evidence to date has suggested an association between sleep duration and osteoporosis risk [18,19,20,21]. In addition, a growing body of evidence has indicated the role of physical activity in osteoporosis prevention [22,23,24]. However, most studies investigating the association between physical activity and osteoporosis prevention only focused on specific types of physical activity, such as walking or sport. Moreover, in the modern life, individuals are grappling with both insufficient physical activity and inadequate sleep. However, the potential synergistic impact of the combination of physical activity and sleep duration on bone density has not been widely explored or discussed; and evidence regarding the combined effects or interaction of sleep duration and physical activity is relatively scarce. Following this context, therefore, we conducted this study to evaluate the role of sleep duration and physical activity and their combination in association with osteoporosis, using a nationally representative database of Taiwan.
Methods
Ethics statement
Taiwan Biobank received ethics approval from the Institutional Review Board on Biomedical Science Research (IRB-BM), Academia Sinica, Taiwan, and from the Ethics and Governance Council (EGC) of Taiwan Biobank, Taiwan. Written informed consent was obtained from each participant in accordance with requirements and the principles of the Declaration of Helsinki. The present study has been approved by the research ethics committee of Taichung Veterans General Hospital (no. CE20023A) and Taiwan Biobank (no. TWBR10901-02).
Data source
Taiwan Biobank Cohort
The Taiwan Biobank enrolled community-based cohort consisting of ~ 120,000 volunteers (as of April 30, 2020) between 30 and 76 years of age with no history of cancer. The Taiwan Biobank is governed by the Ethics and Governance Council (EGC) of the Taiwan Biobank and the Ministry of Health and Welfare, Taiwan.
Each participant completed a physical examination as well as a questionnaire that addressed personal information and lifestyle factors by a face to face interview. Regarding exercise, the questionnaire asks participants whether they have a regular exercise habit (at least 3 times per week, more than 30 min each). If the answer is yes, then the questionnaire asks to list the 3 most frequently performed exercises and their frequency and duration. Based on this information, we calculated metabolic equivalent of tasks (METs)/week of each participants using a conversion formula between types of exercise and the METs. The conversion formula in Taiwan Biobank is shown elsewhere [25]. Participants were classified into 3 groups based on their exercise status (those without regular exercise habit, those with regular exercise habit and intensity less than 20 METs/week, and those with regular exercise habit and intensity more than 20 METs/week).
Study population
The present study extracted data from Taiwan Biobank data cycles. Adults aged > 18 years, had complete information of dual-energy X-ray absorptiometry (DXA) measures on BMD, sleep hours, and components to calculate metabolic equivalent of tasks (METs) were eligible for inclusion. Participants without complete data of body mass index (BMI) were excluded from the primary cohort.
Assessment of sleep duration
Duration of sleep was captured by a single question in the Taiwan Biobank database: “In the past month, how many hours did you sleep on average on weekdays”. Participants were categorized into three groups according to their sleep hours by < 6.5 h/day, 6.5–8 h/day, and ≥ 8 h/day.
Ascertainment of osteoporosis
The study subjects’ BMD at the total femoral areas were measured through DXA scan (Hologic, Bedford, MA, USA). Quality control was routinely conducted on all DXA machines. We categorized the study cohort into with or without osteoporosis, defined as a BMD T-score equal to or less than 2.5 standard deviations (SD) at either the total femur, femoral neck or lumbar spine L1 ~ L4, using reference values from the young Caucasian populations of the same sex according to the WHO criteria.
Assessment of physical activity level
In this study, we used the definition proposed by Ford et al. and the US Department of Health and Human Services for physical activity, which is the sum of the product of the time spent weekly in each activity reported by the participants multiplied by the metabolic equivalent of task (MET) value for that activity, yielding a MET index [26]. One MET was the energy expenditure for every 1 kcal/kg of body weight per hour. Participants were classified into 3 groups based on their METs/week (0; less than 20 METs/week; and ≥ 20 METs/week) in accordance with a previous study [25].
Demography and covariates
Data of participants’ age and gender were obtained from standard questionnaires through in-person interviews conducted by trained interviewers.
Body mass index (BMI) value was obtained from the Taiwan Biobank examination measurements, calculated as body weight (kilograms) divided by height (meters squared). Body weight was measured using an electronic load cell scale, and standing height was measured with a fixed stadiometer. In addition, body fat percentage, waist circumference, hip circumference, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were also extracted from the Taiwan Biobank examination data file.
Level of HbA1c, fasting plasma glucose, serum total cholesterol, triglycerides, high density lipoprotein-cholesterol (HDL-c), low density lipoprotein-cholesterol (LDL-c), GPT, ALT, serum creatinine and uric acid were obtained through the laboratory data.
Diabetes was defined by the question of “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes?” or HbA1c ≥ 6.5% or fasting glucose > 7 mmol/L in laboratory measurements.
GFR was estimated from re-calibrated serum creatinine using the 4-variable Modification of Diet in Renal Disease (MDRD) Study equation. Here we use the IDMS-traceable MDRD Study equation that uses standardized creatinine: GFR = 175 × (standardized serum creatinine)−1.154 × (age)−0.203 × 0.742(if the subject is a woman) × 1.212(if the subject is black). Participants were categorized in to having chronic kidney disease (CKD) with an estimated GFR < 60 ml/min/1.73 m2.
Statistical analysis
Continuous variables are presented in mean and standard deviation (SD). Categorical variables are presented in number and proportion. Analysis of variance (ANOVA) was used for continuous variables to examine the differences of baseline characteristics among the groups of different physical activity. Chi-square test was performed to examine differences in the proportions among the groups of different physical activity. Generalized linear model (GLM) was used to test the joint effects of sleep duration and physical activities on T-score of total femoral area. Logistic regression presented as odds ratio (OR) and 95% confidence interval (CI) was used to test the joint effects of sleep duration and physical activities on risk of osteoporosis. Trend test was also tested by GLM or Logistic regression. All reported p-values were bidirectionally < 0.05 denoting statistical significance.
Results
Study population
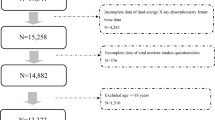
A total of 22,402 subjects had BMD measures by DXA. After excluding individuals who had no complete data of BMI (n = 23), MET score (n = 207), T-score (n = 8,826), and sleep duration (n = 16), 13,330 subjects were included as the primary cohort (Fig. 1).
Characteristics of study population
Table 1 shows the characteristics of the study population. The included subjects were categorized into three groups according to their physical activity levels, and 6,785 subjects (50.90%) had a physical activity level of 0 MET/week, 3,869 subjects (29.02%) had a physical activity level of < 20 METs/week, and 2,676 subjects (20.08%) had a physical activity level of ≥ 20 METs/week. Individuals with a physical activity level of 0 MET/week had higher BMI, bodyweight, body fat percentage, waist circumference, and hip circumference (all p < 0.0001). They also had higher level of triglycerides (p < 0.0001), and ALT (p < 0.0089). However, they had lower levels of SBP, DBP, total cholesterol, HDL-c, GPT, creatinine, and total femur BMD T-score, as well as lower percentage of CKD and DM. The percentage of people with sleep duration < 6.5 h/day and with physical activity level ≥ 20 METs/week (46.11%) was higher than those with a physical activity level < 20 METs/week (42.21%) and zero MET/week (42.79%) (p = 0.0067). The percentage of osteoporosis among participants with physical activity level < 20 METs/week and ≥ 20 METs/week was significantly higher than those who with zero MET (3.6% and 4.73% vs. 4.26%, p = 0.0144). The proportions of participants with a BMD T-score ≤ –2.5 and > –2.5 at different sites are documented in Table 2.
Associations between sleep duration, physical activity and osteoporosis
Table 3 shows the results of univariable and multivariable logistic regression. After adjustment in the multivariable regression, subjects with higher physical activity showed lower risk of having osteoporosis (p for trend < 0.0001). Comparing with those with zero MET/week, only physical level ≥ 20 METs/week showed significantly lower risk of osteoporosis. Comparing with sleep duration 6.5–8 h/day, both higher and lower sleep duration did not show significant lower risk of osteoporosis.
Joint effects of sleep duration and physical activity level in association with osteoporosis
Figure 2 demonstrated the adjusted T-score of total femoral area with variety distribution of physical activity and sleep duration. Physical activity > = 20 METs/week presented higher T-score. Little effects of sleep duration on adjusted T-score was shown in subgroups of physical activity.
However, as shown in Fig. 3, we further analyzed the associations between osteoporosis and sleep duration in view of different physical activity levels. Compared to subjects with a sleep duration < 6.5 h/day and zero MET, individuals with a sleep duration ≥8 h/day and ≥20 METs/week had a significantly lower odds for having osteoporosis (aOR = 0.702, 95% CI = 0.539–0.914). The same tendency can also be found for those with a sleep duration < 6.5 h/day but with an ≥ 20 METs/week (aOR = 0.797, 95% CI = 0.657–0.966). On the contrary, subjects with sleep duration 6.5- 8 h/day with < 20 MET had a non-significantly higher odds for osteoporosis (aOR = 1.129, 95% CI =0.957 –1.331) than the reference group (sleep duration < 6.5 h/day with zero MET). Various combinations of sleep duration and physical activity demonstrated verities of risks of osteoporosis.
Joint effect of sleep hours and physical activity METs in association with osteoporosis. Data are presented aOR and 95%. Reference group: sleep hour < 6.5 h. Multivariable models were adjusted for age, gender, weight, SBP, HbA1c, FPG, total cholesterol, triglycerides, HDL-c, ALT, creatinine, uric acid, and DM
Discussion
In this study, we found that adults with high physical activity levels (≥ 20 METs/week) were 25% less likely to have osteoporosis compared to those with zero MET. On the contrary, adults with an excessive sleep duration ≥ 8 h/day was not independently associated with greater likelihood of osteoporosis than those with normal sleep duration. Adults who had normal sleep duration and a medium physical activity (< 20 METs/week) were not significantly less likely to have osteoporosis compared to short sleepers with no physical activity. Further, in short sleepers, high physical activity level was protective against osteoporosis. Lastly, in those who had zero physical activity, individuals with excessive sleep or short sleep were not significantly likely to have osteoporosis. Together, these findings emphasize the importance of both physical activity and sleep duration in osteoporosis.
The relationship between exercise and BMD was widely documented in the medical literature. Exercise can effectively increase BMD and slow down bone loss especially in middle-aged and older people [22]. Except for the amount of exercise, the frequency and pattern of exercise are all linked to BMD [27, 28]. It has been proposed that exercise can promote bone formation and decrease bone resorption through both genetic and paragenetic pathways [29, 30]. In the present study, consistent with the previous ones, it indicates the independent association between high physical activity and reduced chance of osteoporosis. However, since the previous reports mainly focused on exercise training, our data have certain novelty in providing real-world evidence on routine physical activity measured by METs and its relevance with osteoporosis in a nationwide sample.
Sleep conditions were also linked to BMD. A poor sleep pattern was significantly correlated to reduced BMD when compared to a healthy sleep pattern. Moreover, results of the previous studies revealed that people with later bedtime had a lower femoral BMD and higher risk of osteoporosis than those with normal sleep duration along with usual bedtime [18, 31]. On the other hand, over-sleeping or excess daytime sleep had little effect on BMD [32, 33]. Our study did not specifically assess bedtime or daytime sleep due to lack of data. Nevertheless, the results of present study indicate excessive sleep is not significantly associated with greater likelihood of osteoporosis.
With regard to the potential combined effects of sleep duration and physical activity, our findings show that greater physical activity may be protective against osteoporosis in short sleepers. In addition, it is important to point out that a moderate physical activity (< 20 METs/week) combined with a normal sleep duration (6.5–8 h/day) did not reduce the likelihood of osteoporosis compared to short sleepers who had zero physical activity. To take into account the lifestyle in the modern society, most people can be categorized as having a physical activity level of < 20 METs/week, and our results might suggest that increasing physical activity (≥ 20 METs/week) may be one of the key in lowing the risk of osteoporosis.
Another interesting finding is that when considering the effect of sleep duration and physical activity at the same time, either METs ≥ 20 with a 6.5–8 h’ sleep or ≥ 8h sleep had significantly lower likelihood of osteoporosis compared to short sleepers with no physical activity. This suggest that sleep and physical activity level might have certain interplay and should better be considered together, as a balance of good and bad, in the context of osteoporosis. Among those who have a high physical activity, 6.5 h of sleep may be enough to keep their BMD
Strengths and Limitations
There are two strengths in our study. Firstly, this study evaluated the potential combined effect of sleep duration and physical activity in association with osteoporosis, which there were insufficient evidence in the literature. Secondly, since this study provides explicit epidemiological evidence based on a nationally representative database, the findings are likely generalizable to the overall Taiwanese population.
There are also limitations. This study is of cross-sectional design thus rigid causal inferences cannot be made. We cannot capture whether and how sleep duration and physical activity impact bone loss and the development of osteoporosis by time. Sleep duration and physical activity were identified through interview questionnaires, in which inaccurate reporting or recall bias might have occurred. Sleep quantity and quality would decrease with aging, and aging increases the prevalence of common sleep disorders, which may probably influence the data on sleep duration. These issues might limit the interpretation of the findings in the present analyses. Lastly, there may be unknown confounders not included in the Taiwan Biobank dataset therefore could not be considered in the analyses.
Conclusions
This study presents the first report assessing the joint effects of sleep duration and physical activity METs on T-score and osteoporosis of total femoral area in the general adult population of Taiwan. Excessive sleep is not associated with greater likelihood of osteoporosis, whereas higher physical activity METs was associated with reduced chance of osteoporosis. In short sleepers, high physical activity is associated with lower chance of osteoporosis. Normal sleep duration with enough physical activity also lowers the likelihood of osteoporosis. The findings emphasize the role of both sleep duration and physical activity in association with osteoporosis. Future prospective studies are needed to investigate the interplay between physical activity and sleep duration.
Data availability
Data are available to academic investigators from the authors upon reasonable request.
References
Kimmel DB, Vennin S, Desyatova A et al (2022) Bone architecture, bone material properties, and bone turnover in non-osteoporotic post-menopausal women with fragility fracture. Osteoporos Int 33:1125–1136
Teissier T, Temkin V, Pollak RD et al (2022) Crosstalk between senescent bone cells and the bone tissue microenvironment influences bone fragility during chronological age and in diabetes. Front Physiol 13:812157
Yang K, Cao F, Qiu S et al (2022) Metformin promotes differentiation and attenuates H2O2-induced oxidative damage of osteoblasts via the PI3K/AKT/Nrf2/HO-1 pathway. Front Pharmacol 13:829830
WHO Scientific Group on the Prevention and Management of Osteoporosis (2000: Geneva, Switzerland) (2003) Prevention and management of osteoporosis : report of a WHO scientific group (PDF). pp 7, 31. ISBN 978–9241209212
Jonsson B, Kanis J, Dawson A et al (1999) Effect and offset of effect of treatments for hip fracture on health outcomes. Osteoporos Int 10(3):193–199
Sinnesael M, Claessens F, Boonen S et al (2013) Novel insights in the regulation and mechanism of androgen action on bone. Curr Opin Endocrinol Diabetes Obes 20(3):240–244
Sinnesael M, Boonen S, Claessens F et al (2011) Testosterone and the male skeleton: a dual mode of action. J Osteoporos 2011:1–7
Melton LJ (2003) Epidemiology worldwide. Endocrinol Metab Clin North Am 32(1):v, 1–13
Raisz L (2005) Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115(12):3318–3325
Berg KM, Kunins HV, Jackson JL et al (2008) Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med 121(5):406–418
Zhang F, Zhou F, Liu H et al (2022) Long-term exposure to air pollution might decrease bone mineral density T-score and increase the prevalence of osteoporosis in Hubei province: evidence from China osteoporosis prevalence study. Osteoporos Int. https://doi.org/10.1007/s00198-022-06488-7
Wilson-Barnes SL, Lanham-New SA, Lambert H (2022) Modifiable risk factors for bone health & fragility fractures. Best Pract Res Clin Rheumatol 21:101758
Yoon V, Maalouf NM, Sakhaee K (2012) The effects of smoking on bone metabolism. Osteoporos Int 23:2081–2092
Maurel DB, Boisseau N, Benhamou CL et al (2012) Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int 23:1–16
Hodges JK, Cao S, Cladis DP et al (2019) Lactose intolerance and bone health: the challenge of ensuring adequate calcium intake. Nutrients 11:718
Sarafrazi N, Wambogo EA, Shepherd JA Osteoporosis or low bone mass in older adults: United States, 2017–2018. 2021; NCHS data brief no. 405
Salari N, Ghasemi H, Mohammadi L et al (2021) The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. J Orthop Surg Res 16:609
Zeng H, Li L, Zhang B et al (2022) Relationship between sleep pattern and bone mineral density in patients with osteoporotic fracture. Ther Adv Endocrinol Metab 13:20420188221106884
Daniel S, Cohen-Freud Y, Shelef I et al (2022) Bone mineral density alteration in obstructive sleep apnea by derived computed tomography screening. Sci Rep 12(1):6462
Tang Y, Wang S, Yi Q et al (2021) Sleep pattern and bone mineral density: a cross-sectional study of National Health and nutrition examination survey (NHANES) 2017–2018. Arch Osteoporos 16(1):157
Shiao YC, Chen WT, Chen WL (2021) Association of short sleep duration and trabecular bone score. Sci Rep 11(1):19821
Zhang S, Huang X, Zhao X et al (2022) Effect of exercise on bone mineral density among patients with osteoporosis and osteopenia: a systematic review and network meta-analysis. J Clin Nurs 31(15–16):2100–2111
Little-Letsinger SE, Rubin J, Diekman B et al (2022) Exercise to mend aged-tissue crosstalk in Bone Targeting Osteoporosis & Osteoarthritis. Semin Cell Dev Biol 123:22–35
Otsuka H, Tabata H, Shi H et al (2021) Associations of exercise habits in adolescence and old age with risk of osteoporosis in older adults: the Bunkyo Health Study. J Clin Med 10(24).https://doi.org/10.3390/jcm10245968
Hiraike Y, Yang CT, Liu WJ et al (2021) FTO obesity variant-exercise interaction on changes in body weight and BMI: the Taiwan Biobank Study. J Clin Endocrinol Metab 106(9):e3673–e3681
Ford ES, Wheaton AG, Chapman DP et al (2014) Associations between self-reported sleep duration and sleeping disorder with concentrations of fasting and 2-h glucose, insulin, and glycosylated hemoglobin among adults without diagnosed diabetes. J Diabetes 6(4):338–350
Zitzmann AL, Shojaa M, Kast S et al (2022) The effect of different training frequency on bone mineral density in older adults. A comparative systematic review and meta-analysis. Bone 154:116230
Xu F, Zhang Q, Wang LK et al (2021) Estimates of the effects of physical activity on osteoporosis using multivariable mendelian randomization analysis. Osteoporos Int 32(7):1359–1367
Guo J, Yuan Y, Zhang L et al (2022) Effects of exercise on the expression of long non-coding RNAs in the bone of mice with osteoporosis. Exp Ther Med 23(1):70
Chen X, Yang K, Sun P et al (2021) Exercise improves bone formation by upregulating the Wnt3a/β-catenin signalling pathway in type 2 diabetic mice. Diabetol Metab Syndr 13(1):116
Lee CL, Tzeng HE, Liu WJ et al (2021) A cross-sectional analysis of the association between sleep duration and osteoporosis risk in adults using 2005–2010 NHANES. Sci Rep 11(1):9090
Guo M, Feng T, Liu M et al (2022) Causal roles of daytime sleepiness in cardiometabolic diseases and osteoporosis. Eur Rev Med Pharmacol Sci 26(8):2755–2764
Wu S, Wang P, Guo X et al (2020) The associations between different sleep patterns and osteoporosis based on the osteoporosis self-assessment tool for asians. Arch Osteoporos 15(1):164
Funding
TCVGH-1127313C, TCVGH-1127305D, TCVGH-1117308C, TCVGH-1117305D.
IRB:CE20023A
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Competing interests
All authors declare non-conflict of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Chen, KH., Su, CM., Liu, WJ. et al. The joint effects of physical activity and sleep duration on risk of osteoporosis in Taiwanese adult population: The Taiwan Biobank Study. Osteoporos Int 35, 523–531 (2024). https://doi.org/10.1007/s00198-023-06947-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06947-9