Abstract
Summary
We established a clinical pharmacist adherence management system (CPAMS) led by clinical pharmacists to examine whether denosumab adherence could be improved. The results showed that CPAMS could effectively improve adherence to denosumab and the treatment of osteoporosis. However, this effect weakened during the spread of infectious diseases such as COVID-19.
Purpose
Denosumab is currently one of the drugs that can effectively reduce the risk of clinical fracture. However, as a drug requiring long-term subcutaneous injection, patient adherence to denosumab is the most important factor affecting its therapeutic efficacy. Therefore, we established a clinical pharmacist adherence management system (CPAMS) led by clinical pharmacists and examined whether denosumab adherence could be improved.
Methods
Data were collected from patients receiving denosumab in our hospital between March 2021 and May 2022. The patients who participated in the CPAMS were in the intervention group, and the rest were in the control group. We analysed the proportion of days covered (PDC) value of denosumab, distribution of subsequent visits, and proportion of patients who continued participating during the normal and coronavirus (COVID-19) periods.
Results
Eighty-five patients were enrolled in this retrospective study: 32 in the intervention group and 53 in the control group. The PDC values were significantly higher in the intervention group (0.9875, 0.9025–1) than in the control group (0.5, 0.5–0.5) after 1 year. The subsequent visit rate in the intervention group was 93.80%. However, none of the patients in the control group returned. In the intervention group, the ratio of timely to delayed subsequent visits was 11:19. After the COVID-19 pandemic, the PDC value of the intervention group (0.957, 0.5–1) was lower than that before COVID-19, and the ratio of timely to delayed subsequent visits was 9:13.
Conclusions
Clinical pharmacist-led CPAMS could effectively improve adherence to denosumab and the treatment of osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a chronic metabolic bone disease common among older adults [1]. Since oestrogen depletion causes loss of bone mineral density, postmenopausal women are the main group of patients with osteoporosis [2]. Osteoporosis does not usually show obvious clinical signs until fragility fractures develop. Unfortunately, 80% of female patients are unaware of osteoporosis prevention before diagnosis [3]. Uncontrolled osteoporosis can lead to the recurrence of fragility fractures, eventually leading to disability and even death, resulting in huge economic losses. A study showed that the annual cost of osteoporosis in the USA is expected to reach US$95 billion by 2040 [4].
In healthy young adults, the bone turnover cycle is balanced, with secondary osteoporosis manifesting in the face of chronic disease, aging, and exposures such as glucocorticoids, often attributed to causes like hypogonadism, excessive alcohol intake, and prolonged glucocorticoid use. A common misconception is viewing osteoporosis not as a serious health condition but as a natural consequence of aging. Such perceptions persist despite the potential severe ramifications of untreated osteoporosis. This misconception, combined with the asymptomatic nature of the condition until a fracture occurs, may contribute to the overall poor adherence seen with anti-osteoporosis medications, not just denosumab.
Denosumab is one of the drugs that can effectively reduce the risk of clinical fracture [5]. In 2010, it was the first monoclonal antibody approved for the treatment of osteoporosis. Denosumab binds to and inhibits receptor activator of nuclear factor-κβ ligand (RANKL), which inhibits bone resorption. Long-term (10-year) denosumab treatment has been found to consistently improve bone mineral density and reduce fracture risk in patients without increasing the incidence of adverse events [6]. While adherence to long-term denosumab therapy has been observed to be superior compared to some other osteoporosis treatments like oral bisphosphonates, there is still a notable decline over time that warrants attention [7]. Discontinuation of denosumab can lead to a rapid loss of bone mass in patients, which can greatly increase the risk of multiple vertebral fractures [8, 9].
In this study, we established a clinical pharmacist adherence management system (CPAMS) led by clinical pharmacists and examined whether denosumab adherence could be improved. The performance of the system during the COVID-19 pandemic was also tested.
Methods
Patients
All these patients were admitted to the inpatient department of Ningbo No.6 Hospital from March 2021 to May 2022. Patients aged 50 to 90 years old with lumbar spine or total hip bone mineral density T-scores of less than − 2.5 at either location but greater than –4.0 at both locations and treated with denosumab were included in the study [6]. All patients had their initial denosumab injection prior to discharge, with follow-up injections handled by the outpatient department. Patients who participated in the CPAMS were enrolled in the intervention group, and the remaining patients served as the control group. All patients were educated by clinical pharmacists when they first used denosumab to ensure they were aware of its long-term use (Fig S1).
The study was approved by the Ethics Committee of the Ningbo No.6 Hospital (approval no: X2021034). Formal consent is not required for this study type (retrospective study).
Clinical pharmacist adherence management system (CPAMS)
The CPAMS was established by clinical pharmacists at the Ningbo No.6 Hospital to improve medication adherence. Denosumab was the first drug to be administered using CPAMS. It worked as follows:
After the first use of denosumab, patient information will be included in the Convergence Media doctor-patient mutual action platform (Ningfan Technology, Ningbo) in CPAMS. The CPAMS automatically generates a schedule of denosumab injections every 6 months. One week before the next injection, the platform automatically generated a reminder text message to notify the patients or their families. Simultaneously, the information is synchronised to the clinical pharmacist’s work-related mobile phones. After the patients received subsequent denosumab injections on time, the CPAMS generated a new denosumab injection schedule. If the patient did not visit the clinic on the second day of the scheduled subsequent visit, the clinical pharmacist contacted the patient or their family via telephone to remind them and provide medication consultation services (Fig. 1). All reminder messages were sent in the official name of Ningbo No.6 Hospital. The resulting SMS costs were all borne by the hospital, which is ¥0.01 (approximately $0.0014) per text.
Evaluation method
The proportion of days covered (PDC) value of denosumab, distribution of subsequent visits, and proportion of patients who continued to participate were collected, including during the normal and coronavirus (COVID-19) periods. The COVID-19 period in this study refers to the period of a large number of Chinese people being infected with COVID-19 after China cancelled large-scale nucleic acid testing and quarantine. The COVID-19 period was approximately from December 2022 to February 2023. The PDC values in this study were calculated using the formula: number of days of denosumab coverage in the year after the first dose of denosumab/total number of days (1 year starting with denosumab injection). The PDC value (normal period) was calculated using the time of the first denosumab injection as the starting time, which was recorded as the first PDC. The PDC value (COVID-19 period) was calculated using the time of the second denosumab injection as the starting time, which was recorded as the second PDC. The distribution of subsequent visits included the rate of subsequent visits and the proportion of on-time and delayed visits. Patients who did not return for more than 2 months after the expected subsequent visit were considered to have no intention of continuing denosumab therapy. All enrolled patients were asked if they wished to remain engaged with the CPAMS beyond the duration of the study. This assessed patients’ willingness to continue participating in the system.
Statistical analysis
SPSS version 16.0 (IBM, Armonk, NY, USA) was used for data processing, and statistical significance was set at P < 0.05. Measurement data with a normal distribution were presented as the mean ± SD. Measurement data with non-normal distributions were presented as median (25% percentile, 75% percentile). Count data were presented as percentages. The t-test was used for parametric tests. Kolmogorov–Smirnov and Wilcoxon’s tests were used as non-parametric tests. Differences in constituent proportions were analysed using the chi-square and McNemar’s tests.
Results
A total of 85 patients were enrolled in this study, of which 32 patients had participated in the CPAMS and were included in the intervention group, and the remaining 53 patients were included in the control group. Data on age, sex, education, complications (diabetes), smoking history, and combined medications were collected as baseline characteristics. No significant differences were observed between the intervention and control groups, except for fracture rates (Table 1).
After giving all patients the same denosumab education and follow-up reminders, the first PDC was significantly higher in the intervention group (0.9875, 0.9025–1) than in the control group (0.5, 0.5–0.5) (P < 0.0001) (Fig. 2a). Unexpectedly, none of the patients in the control group returned for a subsequent visit. In contrast, 30 patients (93.75%) in the intervention group returned to the outpatient clinic after discharge (Fig. 2b; Table 2).
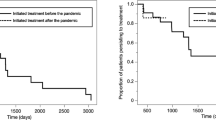
After the COVID-19 pandemic, compared with the first PDC value (0.9875, 0.9025–1), the second PDC value (0.957, 0.5–1) of the intervention group showed a significant reduction (P < 0.0128) (Fig. 3a). The number of patients that returned for subsequent visit decreased from 30 (93.75%) before the COVID-19 outbreak to 22 (68.75%) after the COVID-19 pandemic (Fig. 3b; Table 3).
Notably, the ratio of on-time to delayed subsequent visits was 9:13 in the intervention group after the COVID-19 pandemic, which was not statistically different from 11:19 before the COVID-19 pandemic (P = 0.780) (Fig. 4; Table 3).
At the end of the study, we asked all enrolled patients if they desired to participate in the CPAMS. Thirty-one (96.88%) patients in the intervention group were willing to continue participating in the CPAMS, which was significantly higher than the 19 (35.85%) patients in the control group (P < 0.0001) (Fig. 5; Table 4).
Discussion
Historical data often suggest that prior fractures could enhance adherence to osteoporosis treatments [10]. However, in our cohort, such history did not appear to significantly influence adherence. Despite the higher prevalence of prior fractures in the control group, the adherence rate did not reflect a corresponding increase. In fact, 6 months post-intervention, none of the control group patients returned for medication refills, reinforcing the notion that previous fracture history was not a determinant of adherence in this study context.
In this study, we observed that after 6 months, none of the patients in the control group complied with the doctor’s request for long-term use of denosumab, despite receiving medication education from the clinical pharmacist before discharge from the hospital and during subsequent follow-up visits. This was maintained until the end of the study period. This suggests that adherence issues with denosumab therapy could be more pronounced in China compared to other nations. There are many factors that affect adherence, including patient preferences, cultural differences, communication with physicians, and pharmacists' education [11]. In China, there is a general perception that one should “avoid taking drugs as much as possible to avoid adverse reactions.” Long-term drug use is not easily accepted by Chinese people, especially the older population. A study conducted in other countries also showed that patient concerns about long-term safety (30.3%) and experience of side effects (29.7%) were significant factors for discontinuation [12]. Similar patterns of medication discontinuation or non-adherence have been observed with antirheumatic and anti-gout drugs [13, 14]. This overarching sentiment towards medication might explain the results presented at the beginning of this paragraph.
We acknowledge the pivotal role shared decision-making plays in enhancing patient compliance. Engaging patients in their treatment decisions fosters intrinsic motivation, reinforcing adherence [15]. Although we did not implement a structured SDM process, the cost of denosumab necessitated patients being well-informed by their physician and clinical pharmacist, with alternative treatment options presented. Consequently, those who opted for denosumab did so voluntarily. This, we believe, emulates a simplified SDM approach. However, the fluid nature of patient decisions over a 6-month span was evident, as many shifted their focus from osteoporosis treatment. For several patients, osteoporosis was perceived as a mere age-related phenomenon, minimizing its potential risks. They felt caution in daily activities would suffice to prevent future fractures [16]. Intriguingly, despite SMS reminders, a significant proportion of patients in the intervention group still required the direct intervention of clinical pharmacists, which effectively became an iterative shared decision-making process.
In addition, forgetting was hypothesised to be another cause of poor adherence. Forgetfulness can arise from various factors such as managing multiple commitments and the responsibility of taking several medications. Handling numerous medication schedules can amplify the complexity of personal healthcare management, potentially leading to oversight or missed doses [17]. Therefore, CPAMS was designed to inform the patient 1 week prior to the next injection. Based on the results of the intervention group, the early reminder text message was considered effective. Mobile telephone text messaging has been shown to improve medication adherence in middle-aged patients with chronic diseases [18]. However, approximately one-third of the patients in the intervention group met the expected subsequent visit time. This proportion increased after the COVID-19 pandemic; however, the difference was not statistically significant. The remaining patients required second telephone reminders from the clinical pharmacists. In communication with the clinical pharmacist, some patients said they forgot the injection time again, and some did not notice the initial reminder. Automated reminder text messages sent by the CPAMS are of limited use. Relevant literature has also pointed out that without the support of medical staff, it is difficult to improve the effects of medication reminders alone [19, 20]. In addition, all patients who communicated with clinical pharmacists were asked about the side effects of the long-term use of denosumab. Secondary medication education by clinical pharmacists appeared to be the key to CPAMS improving adherence to denosumab. However, the workload and cost of clinical pharmacists conducting multiple education or follow-up sessions for each patient need to be considered, especially in China, where there is a shortage of clinical pharmacists.
Clinical pharmacist interventions are effective in improving medication adherence. The interventions include medication education and telephone or face-to-face follow-up [21,22,23]. Several studies have shown that clinical pharmacists effectively improve treatment effects in managing chronic hypertension [21, 22, 24]. The approach of pharmacy student-based interventions to reduce the burden on clinical pharmacists has been proven effective [25]. However, considering the time cost and low professionalism of students, we believe it still needs improvement. Therefore, the CPAMS was designed so that it did not require constant attention from clinical pharmacists once a patient entered the system. When the time for the next injection approaches, the system alerts both the patient and the clinical pharmacist via mobile phone text messages. At this point, the clinical pharmacist pays more attention to the patient. The purpose of this function is to prevent clinical pharmacists from forgetting. If the SMS reminder for the patient fails, the clinical pharmacist sends a second reminder and provides medication education. Older patients may prefer telephone follow-ups by clinical pharmacists because of factors such as a low education level or vision. Additionally, real-time communication appears to be more memorable than text-message communication. In this study, approximately two-thirds of the patients received a second reminder from their clinical pharmacist and returned for a subsequent visit.
The COVID-19 pandemic greatly challenged the healthcare services of all countries affected [26]. In this study, we observed that the improvement effect of CPAMS was weakened under the impact of the COVID-19 pandemic, which specifically manifested as a reduction in the second PDC value. In fact, China’s regional lockdown policy during the initial stages of the COVID-19 pandemic did not cause much interference with CPAMS, and patients with osteoporosis with adequate protection were willing to visit the hospital. However, after the cancellation of the prolonged lockdown in China, the COVID-19 infection began to spread [27, 28]. The rapid increase in the number of patients infected with COVID-19 was the main reason patients with osteoporosis were unwilling to go out. This explains why the second PDC value was significantly lower in the intervention group. However, although the COVID-19 pandemic inevitably affected the operation of CPAMS, more than 50% of patients in the intervention group completed continuous denosumab treatment, which was still much higher than that in the control group. Interestingly, 59.09% of the patients who completed the follow-up visit still required secondary reminders and drug education from clinical pharmacists. This suggests that the role of clinical pharmacists in CPAMS is important.
There is no universal measure of medication adherence. Direct measurements, such as blood concentration monitoring, are accurate but expensive and difficult to implement. Current measures of adherence include the medication possession ratio (MPR), proportion of days covered (PDC), pill count, clinician assessment, and patient self-report [29]. In this study, all denosumab injections were administered in the outpatient clinic of the hospital by a nurse. Therefore, the PDC value was a more appropriate index for evaluating adherence in this study. To improve the health outcomes of the patients, a uniform reminder of denosumab injection administration was initiated by the clinical pharmacist at the end of the study for all patients, including those in the control group. The willingness of patients to participate in CPAMS in the future rather than creating questionnaires or questions and answers was a better indicator of patient satisfaction with CPAMS, given the need to prevent patients from feeling oppressed or the difficulty of understanding in older patients [30]. Patients only needed to answer whether they were interested in participating subsequently; this avoids taking up too much time, which can cause impatience. All patients in the intervention group were willing to continue participating in the CPAMS, except for one who discontinued denosumab because of financial problems. However, only 19 patients in the control group expressed willingness to participate in the system. We found that the acceptance of CPAMS was much higher in patients who participated continuously than in those who did not. This suggests that CPAMS intervention should be initiated at the initial time of drug use and continued thereafter.
A limitation of this study is that the precise reasons for the discontinuation of denosumab were not studied to minimise the discontinuation. The study’s power was limited by the few participating patients, as CPAMS, still in validation, was approved for limited use in select departments. In addition, the results of patient satisfaction with CPAMS were too simple, and there was a lack of multidimensional evaluation for improvement. Finally, the current CPAMS, which only improves adherence to denosumab, is inadequate. Therefore, using CPAMS to improve adherence to other drugs, such as anti-gout drugs, will be the direction of our next study.
Conclusion
The clinical pharmacist-led CPAMS could effectively improve denosumab adherence and the treatment of osteoporosis. Although this effect weakened during the spread of infectious diseases such as COVID-19, there was still improvement. Most patients were satisfied with the system and willing to continue participating.
Data Availability
All relevant data is contained within the article: The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
References
Osteoporosis GV (2023) Osteoporosis. Med Clin North Am 107(2):213–225
Compston JE, McClung MR, Leslie WD (2019) Osteoporosis. Lancet (London, England) 393(10169):364–376
Angum F, Khan T, Kaler J, Siddiqui L, Hussain A (2020) The prevalence of autoimmune disorders in women: a narrative review. Cureus 12(5):e8094
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Mineral Res 22(3):465–475
Ayers C, Kansagara D, Lazur B, Fu R, Kwon A, Harrod C (2023) Effectiveness and safety of treatments to prevent fractures in people with low bone mass or primary osteoporosis: a living systematic review and network meta-analysis for the American College of Physicians. Ann Intern Med 176(2):182–195
Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR et al (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5(7):513–523
Borek DM, Smith RC, Gruber CN, Gruber BL (2019) Long-term persistence in patients with osteoporosis receiving denosumab in routine practice: 36-month non-interventional, observational study. Osteoporos Int 30(7):1455–1464
Gonzalez-Rodriguez E, Aubry-Rozier B, Stoll D, Zaman K, Lamy O (2020) Sixty spontaneous vertebral fractures after denosumab discontinuation in 15 women with early-stage breast cancer under aromatase inhibitors. Breast Cancer Res Treat 179(1):153–159
Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O (2017) Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Mineral Res 32(6):1291–1296
Tremblay É, Perreault S, Dorais M (2016) Persistence with denosumab and zoledronic acid among older women: a population-based cohort study. Arch Osteoporos 11(1):30
McQuaid EL, Landier W (2018) Cultural issues in medication adherence: disparities and directions. J Gen Intern Med 33(2):200–206
Lindsay BR, Olufade T, Bauer J, Babrowicz J, Hahn R (2016) Patient-reported barriers to osteoporosis therapy. Arch Osteoporos 11(1):19
van den Bemt BJ, van den Hoogen FH, Benraad B, Hekster YA, van Riel PL, van Lankveld W (2009) Adherence rates and associations with nonadherence in patients with rheumatoid arthritis using disease modifying antirheumatic drugs. J Rheumatol 36(10):2164–2170
Singh JA (2014) Facilitators and barriers to adherence to urate-lowering therapy in African-Americans with gout: a qualitative study. Arthritis Res Ther 16(2):R82
Milky G, Thomas J 3rd (2020) Shared decision making, satisfaction with care and medication adherence among patients with diabetes. Patient Educ Couns 103(3):661–669
Watts NB, Manson JE (2017) Osteoporosis and fracture risk evaluation and management: shared decision making in clinical practice. JAMA 317(3):253–254
Chowdhury T, Dutta J, Noel P, Islam R, Gonzalez-Peltier G, Azad S et al (2022) An overview on causes of nonadherence in the treatment of rheumatoid arthritis: its effect on mortality and ways to improve adherence. Cureus 14(4):e24520
Thakkar J, Kurup R, Laba TL, Santo K, Thiagalingam A, Rodgers A et al (2016) Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med 176(3):340–349
Choudhry NK, Krumme AA, Ercole PM, Girdish C, Tong AY, Khan NF et al (2017) Effect of reminder devices on medication adherence: the REMIND randomized clinical trial. JAMA Intern Med 177(5):624–631
Kini V, Ho PM (2018) Interventions to improve medication adherence: a review. JAMA 320(23):2461–2473
Ho PM, Lambert-Kerzner A, Carey EP, Fahdi IE, Bryson CL, Melnyk SD et al (2014) Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Intern Med 174(2):186–193
Stewart K, George J, Mc Namara KP, Jackson SL, Peterson GM, Bereznicki LR et al (2014) A multifaceted pharmacist intervention to improve antihypertensive adherence: a cluster-randomized, controlled trial (HAPPy trial). J Clin Pharm Ther 39(5):527–534
Hedegaard U, Kjeldsen LJ, Pottegård A, Henriksen JE, Lambrechtsen J, Hangaard J et al (2015) Improving medication adherence in patients with hypertension: a randomized trial. Am J Med 128(12):1351–1361
Magid DJ, Ho PM, Olson KL, Brand DW, Welch LK, Snow KE et al (2011) A multimodal blood pressure control intervention in 3 healthcare systems. Am J Manag Care 17(4):e96-103
Tamargo C, Sando K, Prados Y, Cowart K (2019) Change in proportion of days covered for statins following implementation of a pharmacy student adherence outreach program. J Manag Care Spec Pharm 25(5):588–592
Minisola S, Cipriani C, Vigna E, Sonato C, Colangelo L, Monti F et al (2022) COVID pandemic and denosumab adherence. Osteoporos Int 33(4):943–944
National Health Commission of the People's Republic of China (2022) Notice on Further optimizing and implementing the prevention and control measures of COVID-19 epidemic. National Health Commission of the People’s Republic of China [Available from: http://www.nhc.gov.cn/xcs/zhengcwj/202212/8278e7a7aee34e5bb378f0e0fc94e0f0.shtml. Accessed 07 Dec 2022
Chinese Center for Disease Control and Prevention (2023) Epidemic situation of 2019 novel coronavirus infection in China. China Center for Disease Control and Prevention [Available from: https://www.chinacdc.cn/jkzt/crb/zl/szkb_11803/jszl_13141/202304/t20230401_264798.html. Accessed 01 Apr 2023
Lam WY, Fresco P (2015) Medication adherence measures: an overview. Biomed Res Int 2015:217047
Farmer KC (1999) Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clin Ther 21(6):1074–90 (discussion 3)
Acknowledgements
We thank all patients in this study. We thank all of our colleagues in the hospital who have been involved in the patient’s care and research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in the study were in accordance with the ethical standards of the Ningbo NO.6 Hospital (approval no: X2021034) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yang, Q., He, J. & Yuan, F. Improvement of proportion of days covered for denosumab under implementation of clinical pharmacist adherence management system: normal and COVID-19 period. Osteoporos Int 35, 309–316 (2024). https://doi.org/10.1007/s00198-023-06933-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06933-1