Abstract
Summary
In this multi-institutional retrospective cohort study, we compared the long-term risk of osteonecrosis of the jaw following the use of denosumab vs. bisphosphonates in osteoporotic patients. After 2-year use, the likelihood of osteonecrosis of the jaw is lower with denosumab compared to bisphosphonates, and the difference increases with time.
Purpose
To compare the long-term risk of osteonecrosis of the jaw (ONJ) between osteoporotic patients treated with bisphosphonates (BPs) and denosumab.
Methods
This multi-institutional retrospective cohort study included patients aged > 40 years with osteoporosis between January 2010 and December 2018. Patients who met the eligibility criteria were divided into BPs and denosumab groups by propensity score matching (PSM). The risk of ONJ of denosumab vs. BPs was estimated using a Cox proportional hazards model and was described by the cumulative incidence rate using the Kaplan–Meier method.
Results
A total of 84,102 patients with osteoporosis were enrolled, among whom, 8962 were eligible for inclusion based on their first-line drug use (denosumab, n = 3,823; BPs, n = 5,139). Following PCM matching (1:1), the BPs and denosumab groups included 3665 patients each. The incidence density of ONJ in the denosumab and BPs matching groups was 1.47 vs. 2.49 events (per 1000 person-years), respectively. The hazard ratio of ONJ in the denosumab vs. BPs group was estimated as 0.581 (95% confidence interval: 0.33–1.04, p = 0.07). The cumulative incidence rates of ONJ in both groups were similar for the first and second years of drug use (p = 0.062), but significantly different from the third year onwards (p = 0.022). The severity of ONJ was not significantly different between the two groups.
Conclusion
In osteoporotic patients, after 2 years of use, the likelihood of ONJ being induced by denosumab is lower than that of BPs, and the difference increases with time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 10 million people in the USA have osteoporosis[1]. Osteoporosis-related fractures can increase disability, total health care costs, and mortality[2]. Many treatments can be used to treat osteoporosis depending on its severity. Bisphosphonates (BPs) and denosumab have been widely used to treat osteoporosis since 1995 and 2010, respectively. However, patients sometimes experience osteonecrosis of the jaw (ONJ) as a result of taking BPs and denosumab [3, 4]. ONJ is a persistent painful necrosis of bone in the maxillofacial region, which is associated with significant morbidity and reduced quality of life, although its pathogenesis remains poorly understood [5]. BPs are a class of antiresorptive medications, which were first introduced to the American market in 1995 to treat bone diseases such as bone cancer, metabolic bone diseases, and osteoporosis. In 2003, a handful of case studies reported the occurrence of ONJ following BPs use [6, 7]. In 2007, the American Association of Oral and Maxillofacial Surgeons (AAOMS) provided a definition for bisphosphonates-related osteonecrosis of the jaw (BRONJ) [8]. In 2014, the term was revised to medication-related osteonecrosis of the jaw (MRONJ) given the finding that it was also associated with the use of other antiresorptive agents (denosumab) or angiogenesis inhibitors (bevacizumab) [9]. Risk factors for MRONJ include age [10], sex, comorbidities (diabetes mellitus [DM] [5], chronic kidney disease [CKD], anemia [11], hyperthyroidism [12], hypothyroidism, rheumatoid arthritis, periodontal disease [11, 13]), dental treatment regimens (tooth extractions [11], ill-fitting dentures), and drugs (corticosteroid [14], antiangiogenic agents [bevacizumab and sunitinib] [15]). The incidence of MRONJ among patients using BPs injections has been reported as 2 to 15% [16]. In a survey study of more than 13,000 Kaiser Permanente members, the prevalence of MRONJ in osteoporotic patients receiving long-term oral BP therapy was reported as 0.1% (10 cases per 10,000), which increased to 0.21% (21 cases per 10,000) in patients who had been taking oral BPs for more than 4 years [17]. Moreover, patients undergoing dental treatment have been shown to have a higher risk of developing MRONJ. Another study that analyzed patients with osteoporosis who were exposed to yearly BPs (Zoledronate®) therapy over a 3-year period reported a MRONJ risk of 0.017% (1.7 cases per 10,000 patients) [18], while an extension of this study to 6 years showed no change in the frequency of MRONJ [19].
Denosumab, a human receptor activator of nuclear factor kappa-B ligand (RANKL) monoclonal antibody, with a different mechanism from BPs, was approved for commercial use in the USA in 2010 and in Taiwan in 2011 (Taiwan Ministry of Health and Welfare, 2011). Preliminary clinical results [20, 21] have shown that denosumab has comparable effects to BPs in treating osteoporosis and bone mass damaged by cancer [20,21,22,23,24,25,26,27]. In 2010, Aghaloo et al. [28] first reported a case of ONJ related to the use of denosumab in a patient with a sacral giant cell tumor. Although clinical cases of MRONJ have been reported, the mechanisms by which denosumab acts on the bone differ from those of BPs [29,30,31,32]. Given its 28-day half-life, it is believed that denosumab will not be retained in the body for long periods, and short-term follow-ups have demonstrated its low likelihood of inducing MRONJ [20, 21, 33]. Recent studies on osteoporotic patients exposed to denosumab have reported a risk of MRONJ of 0.04% (4 cases per 10,000 patients) [21]. Some studies have reported that the incidence of MRONJ among patients with cancer exposed to high doses of denosumab ranges from 0.7 [34] to 1.9% [35]. Therefore, the incidence of denosumab-related ONJ (DRONJ) does not differ significantly from that of BPs-related ONJ in patients with cancer [36, 37]. However, the results vary across countries and populations. Additionally, there is no consensus on whether denosumab treatment poses a lower risk of MRONJ in osteoporosis patients with long-term administration. The incidence difference of MRONJ in previous studies could be due to the difference in MRONJ risk factors and populations in these studies. Moreover, early studies focused on European and North American countries, which differ from Asian countries in terms of race, economic condition, medical status, and patient-related factors. Indeed, a recent study by Taguchi [16] revealed that the incidence of MRONJ is higher in Asian countries, highlighting the importance of conducting large-scale research on this issue in the Asian region [38,39,40,41,42,43].
Therefore, the objective of this study was to compare the risk of MRONJ between osteoporosis patients using BPs vs. denosumab based on data collected from the private database of multiple large-scale medical institutions, called the Chang Gung Research Database (CGRD) [44, 45]. This database accounts for 1/10 of Taiwan’s National Health Insurance Database (NHIRD). The NHIRD was considered as a reference because it covers all medical claims from 99.9% of the entire Taiwanese population. We also validated whether the long-term use of denosumab poses a lower risk of MRONJ than the long-term use of BPs. Finally, the severity of MRONJ was classified based on the treatment regimens to better understand whether differences exist in the severity of MRONJ following the use of BPs and denosumab. These results are expected to serve as a reference for clinicians to administer suitable medications for patients with osteoporosis.
Methods
Study design and population
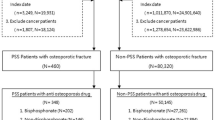
We conducted a multi-institutional retrospective cohort study using the Chang Gung Research Database (CGRD) [44, 45]. The study population comprised patients who were diagnosed with osteoporosis between January 2010 and December 2018. The ICD-9-CM and ICD-10-CM diagnosis codes for osteoporosis are 733.0 and 733.1, respectively. The exclusion criteria were as follows: (1) < 40 years old; (2) previously received radiotherapy; (3) diagnosed with cancer; (4) diagnosed with ONJ before being diagnosed with osteoporosis; (5) diagnosed with ONJ before starting anti-osteoporotic drugs; (6) < 1 year of continuous anti-osteoporotic drug use; (7) no drug use within a year prior to ONJ diagnosis; (8) failure to attend follow-ups after seeking treatment; and (9) used drugs for osteoporosis other than BPs or denosumab or did not seek treatment at all. The eligible patients were divided into BPs and denosumab groups based on their choice of drug (Fig. 1).
Co-variables and propensity score matching
The patients’ age, sex, comorbidities, use of other drugs, and dental treatment regimens were recorded and analyzed in both groups given that these are relevant factors on ONJ. Propensity score matching was used for group matching to diminish the influence of relevant factors on ONJ in group comparison. The variables included in the matching process were age, sex, comorbidities (DM, CKD, anemia, hyperthyroidism, hypothyroidism, rheumatoid arthritis), dental treatment regimen, and drugs (steroids). After matching both groups at a 1:1 ratio, the incidence of ONJ and the correlation between the duration of drug use and the occurrence of ONJ were derived. The severity of ONJ was assessed based on the dental treatment regimen that was used.
Outcome measures: ONJ complication
All of the patients in both groups had continuously and exclusively used the respective drug for more than 1 year. We evaluated the occurrence of ONJ in each group after the use of anti-osteoporotic drugs from January 2010 to December 2018. The occurrence of ONJ was confirmed by the ICD-9-CM and ICD-10-CM diagnosis codes and the related dental procedure (Appendix).
Statistical analysis
Descriptive statistics are expressed as the mean ± standard deviation (SD), median (range), or frequency (percentage), as appropriate. All numerical variables were tested with normality by P-P plots or Kolmogorov–Smirnov test. Data were log-transformed to the approximate normal distribution with the right skewness. Student’s t-test was used to compare continuous variables between groups, and the chi-squared test or Fisher’s exact test was used to compare categorical variables between groups. We estimated the propensity score by modeling the probability of being in the BPs group vs. the denosumab group. The cumulative incidences of ONJ were described by the Kaplan–Meier method. The differences in the time to ONJ development were compared with the log-rank test. The Cox proportional hazards regression model was employed to analyze the hazard ratio of the associated factors for ONJ development. A two-tailed p value < 0.05 indicated statistical significance. All statistical analyses were performed with SPSS version 20.0 (SPSS Inc, Chicago).
Results
Based on the private database of the large-scale medical institution (CGRD), 84,102 patients were diagnosed with osteoporosis between January 2010 and December 2018. We excluded 2463 patients who were < 40 years; 1,515 patients who had received head and neck radiotherapy before enrollment; 2764 patients who were diagnosed with cancer before enrollment; 612 patients with ONJ occurrence before osteoporosis; 138 patients with ONJ occurrence before the use of osteoporosis drugs; 43,693 patients who were using drugs other than BPs or denosumab or did not seek treatment at all; 20,334 patients who did not continuously use anti-osteoporotic drugs more than 1 year; and 3621 patients who failed to attend follow-ups after seeking treatment. After excluding 75,140 patients, the 8962 eligible patients were divided into two groups based on their drug use; the BPs group included 5139 patients, and the denosumab group included 3823 patients (Fig. 1). Following propensity score matching at a 1:1 ratio, each matching group included 3665 patients. The two groups showed no significant differences in related systemic diseases and drug use, but certain factors, such as tooth extraction, were significantly higher percentages in the denosumab group (p < 0.04) (Table 1). After matching, the incidence of ONJ in both groups was calculated, along with the correlation between the duration of drug use and MRONJ occurrence, and the severity of MRONJ was assessed based on the dental treatment regimen used (Table 2). The incidence density of ONJ in the BPs and denosumab groups was 2.49 and 1.47 (per 1000 person-years), respectively. The hazard ratio (95% confidence interval) of denosumab vs. BPs was estimated as 0.581 (0.33–1.04) (p = 0.07). Regarding the correlation between the duration of drug use and ONJ occurrence, the cumulative incidence rates of ONJ in both groups were similar for the first and second years after drug use, but significant differences were observed from the third year onwards (Figs. 2, 3). Moreover, the likelihood of ONJ occurrence stabilized over time in the denosumab group but increased in the BPs group. As shown in Fig. 2, the cumulative incidence rate of ONJ in the BPs group was approximately twofold more than that of the denosumab group from the fourth year onwards. The severity of MRONJ comprises three stages, each with its own treatment approach: Stage 1 involves drug treatments; stage 2 involves local debridement; and stage 3 involves surgical debridement and resection [46]. Even though there was little difference between the BPs and denosumab groups in terms of the severity of ONJ, in the denosumab group, 13 of 18 patients with ONJ received drug treatment, while the remaining five patients received surgical debridement and resection to remove large areas of damaged tissue; in contrast, most patients with ONJ in the BPs group received drug treatment and local debridement. Those differences were not statistically significant in the severity of ONJ (Table 2).
The clinical characteristics of patients with ONJ receiving different antiresorptive agents are shown in Table 3. In the denosumab group, patients with ONJ had a significantly higher incidence of hypothyroidism (p = 0.025), tooth extraction (p = 0.018), and steroid use (p = 0.041), and were younger (p = 0.048) than those patients without ONJ. In the BPs group, patients with ONJ had a significantly higher incidence of DM (p = 0.035), tooth extraction (p < 0.001), deep periodontal curettage (p < 0.001), and steroid use (p = 0.044).
Discussion
The results of this study demonstrated that after a 2-year use, the likelihood of ONJ being induced by denosumab use was lower than that of BPs use for osteoporosis patients, and the difference increased with time. However, there was no significant difference in the severity of MRONJ between the two treatment groups.
In our study, the incidence density of BRONJ was 2.49 (per 1000 person-years). Among previous studies in osteoporosis patients in Asia, the closest value reported was 2.83 (per 1000 person-years), which was reported in the National Taiwan University study by Chiu [43]. However, remarkable differences existed in other Asian studies, with reported values ranging from 0.08 to 2.8 (per 1000 person-years) [16]. Therefore, the value obtained in our study was within a reasonable range in Asia. The range of incidence rate or density reported in previous studies may be due to the inclusion of different countries, evaluated years, ONJ case definitions, and risk factors. Moreover, in previous studies, there seems to be a tendency that the more years evaluated, the higher the incidence density of BRONJ.
In this study, the likelihood of developing ONJ following the long-term use of denosumab is lower than that of BPs (1.47 vs. 2.49 per 1000 person-years, respectively). However, the likelihood of ONJ occurrence as a result of the exclusive use of low-dose denosumab for treating osteoporosis is lower than that reported for cancer treatment [39]. Moreover, a report by Khan [5] revealed that the incidence density of DRONJ was 0 to 30.2/100,000 patient-year in osteoporotic patients [20, 21, 47]. Previous studies were mostly conducted on a small scale with small sample sizes, which contributed to the low incidence of ONJ reported in those studies. Short-term studies are also less able to reflect the cumulative effects of drugs on ONJ incidence. To the best of our knowledge, no previous study has conducted a long-term head-to-head comparison of BPs vs. denosumab in patients with osteoporosis. Moreover, we used propensity score matching with a 1:1 ratio to increase the comparability between groups using different antiresorptive medications (BPs vs. denosumab). In consideration of the time effect, survival analysis was used with a Cox proportional hazards regression model and the Kaplan–Meier method. As our study was based on 8 years of data from multiple large-scale medical institutions, the likelihood of developing BRONJ or DRONJ is likely to be higher than that reported in previous studies. Despite this, the likelihood of MRONJ being induced by low doses of denosumab is still lower than that of long-term BPs. Additionally, differences were observed between the duration of drug use and ONJ occurrence. The cumulative incidence rate of ONJ in the first and second years of drug use were very similar (p = 0.062, log-rank test), but remarkable differences began to surface from the third year onwards (p = 0.022, log-rank test). The patients in the BPs group were twice as likely to develop ONJ compared to those in the denosumab group after the third year of drug use (Fig. 2). The likelihood of DRONJ occurrence in this study was five-fold higher than that reported in previous studies [5]. Therefore, when time is considered, the likelihood of developing ONJ following long-term use of denosumab is lower than that following BPs use, which could be due to the relatively low cumulative effect caused by the mechanisms of action of denosumab. Additionally, in Taiwan, the National Health Insurance scheme and the high accessibility of healthcare resources may contribute to the higher incidence density due to earlier detection and a higher treatment rate, in contrast to patients in other countries, who, when presenting with mild symptoms of ONJ, tend to not seek treatment due to the difficult health insurance environment.
ONJ case definitions were inconsistent before 2007 due to the lack of a specific diagnosis code. In 2007, the AAOMS proposed a case definition of BRONJ based on existing literature and clinical observations [8]. However, the adoption rate of these criteria in clinical practice is unknown. Although the ONJ diagnosis code (ICD-9: 733.45) was used after 2007, Solomon et al. [48] found that most patients with this diagnosis code did not actually have ONJ and that many cases reported an osteoporosis-related fracture instead of ONJ until 2010. Hence, we confirmed the occurrence of ONJ by both the potential ICD diagnosis codes and the related dental procedure (Appendix).
At present, there is no consensus on the treatment of ONJ, with some physicians preferring a conservative approach, and others preferring aggressive methods. According to the guidance from the AAOMS [49], the management strategies of MRONJ are based on the severity of MRONJ (MRONJ staging 0–3) and its progression. The choice of management strategies, either nonoperative or operative therapies, are based on patient factors and surgical judgement in a shared decision-making model with the patient, their family, and medical and dental providers, which should be patient-specific and tailored to individual needs. Nonoperative therapies were documented to be useful in all stages and led to cures in earlier stages or disease stabilization, which included patient education, pain control, secondary infection control, and sequestration of the exposed necrotic bone [49, 50]. In stage 1, patients could use chlorhexidine to improve oral hygiene by chemically removing biofilm from the necrotic bone surface [50]. Surgery is not indicated in stable cases with an adequate quality of life. In stage 2, patients may experience difficulty with local wound care and may require antibiotics to control symptoms. In stage 2 or 3, patients who are poor surgical candidates may be indicated for nonoperative therapies [50]. Nonoperative therapies do not always result in the sequestration of the exposed necrotic bone with disease resolution [51]. Thus, operative interventions, including marginal or segmental resection or partial maxillectomy, should be presented as a treatment option to reduce the progression of disease and can be applied to patients with all stages of MRONJ [52]. In patients with more advanced disease at presentation or progressive clinical or radiographic aspects, surgical resections should be performed; these resections require margins beyond the borders of the necrotic bone to an area of vital bone [53]. Based on the data in this study, there are no significant differences in the severity of ONJ induced by BPs and denosumab. Even though surgery is more effective than conservative treatment [54, 55], the patient’s psychological and physiological conditions must be taken into account whenever necessary, which is why different patients were subjected to different treatment approaches.
The histopathological and radiologic features varied between patients receiving denosumab and those receiving BPs [56]. For instance, denosumab-related ONJ showed significantly lower numbers of osteocytes per area. Additionally, BPs-related ONJ showed numerous bone resorption lacunae on the necrotic bone surface [57], which were limited in denosumab-related ONJ. Radiologic features of BPs-related ONJ are clearly described in the literature [58] and include thickened lamina dura, bone sclerosis, prominence of the inferior alveolar canal, and pathological fractures, in addition to the features of sequestrum, subperiosteal bone formation, and lysis of the cortical border of the jaw [59].
Regarding the limitations of our research, as the CGRD database was used in this study, data on the drugs taken by the patients that were not administered by Chang-Gung medical institutions were not accounted for. Moreover, stage I ONJ cases may not be detected due to mouthwash use alone without oral antibiotic use and dental procedure [8]. Furthermore, as the incidence density of DRONJ was calculated in the propensity-score matching group, the matching process may produce selection bias, leading to a misestimated incidence density of DRONJ. However, the study by Lin [42] showed that the incidence densities were similar between groups before and after propensity-score matching.
In conclusion, this is the first Asian study to provide a head-to-head comparison of the risk of ONJ with BPs vs. denosumab in osteoporotic patients using a multi-institutional retrospective cohort study in Taiwan. Our results demonstrated that after 2-year use, the likelihood of ONJ being induced by long-term denosumab is lower than that of BPs, and the difference increases with time. These findings have the potential to directly impact the therapeutic strategy employed in patients with osteoporosis.
References
Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B (2014) The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29:2520–2526
Camacho PM, Petak SM, Binkley N et al (2016) American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis - 2016. Endocr Pract 22:1–42
Bansal H (2022) Medication-related osteonecrosis of the jaw: An update. Natl J Maxillofac Surg 13:5–10
Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F, American Association of O, Maxillofacial S (2014) American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg 72:1938–1956
Khan AA, Morrison A, Hanley DA et al (2015) Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res 30:3–23
Marx RE (2003) Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 61:1115–1117
Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL (2004) Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 62:527–534
Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws AAoO, Maxillofacial S (2007) American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg 65:369–376
Greuter S, Schmid F, Ruhstaller T, Thuerlimann B (2008) Bevacizumab-associated osteonecrosis of the jaw. Ann Oncol 19:2091–2092
Yamazaki T, Yamori M, Yamamoto K, Saito K, Asai K, Sumi E, Goto K, Takahashi K, Nakayama T, Bessho K (2012) Risk of osteomyelitis of the jaw induced by oral bisphosphonates in patients taking medications for osteoporosis: a hospital-based cohort study in Japan. Bone 51:882–887
Barasch A, Cunha-Cruz J, Curro FA et al (2011) Risk factors for osteonecrosis of the jaws: a case-control study from the CONDOR dental PBRN. J Dent Res 90:439–444
Thumbigere-Math V, Tu L, Huckabay S, Dudek AZ, Lunos S, Basi DL, Hughes PJ, Leach JW, Swenson KK, Gopalakrishnan R (2012) A retrospective study evaluating frequency and risk factors of osteonecrosis of the jaw in 576 cancer patients receiving intravenous bisphosphonates. Am J Clin Oncol 35:386–392
Tsao C, Darby I, Ebeling PR, Walsh K, O’Brien-Simpson N, Reynolds E, Borromeo G (2013) Oral health risk factors for bisphosphonate-associated jaw osteonecrosis. J Oral Maxillofac Surg 71:1360–1366
Saad F, Brown JE, Van Poznak C et al (2012) Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol 23:1341–1347
Guarneri V, Miles D, Robert N, Dieras V, Glaspy J, Smith I, Thomssen C, Biganzoli L, Taran T, Conte P (2010) Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat 122:181–188
Taguchi A, Shiraki M, Morrison A, Khan AA (2017) Antiresorptive agent-related osteonecrosis of the jaw in osteoporosis patients from Asian countries. Osteoporos Sarcopenia 3:64–74
Lo JC, O’Ryan FS, Gordon NP et al (2010) Prevalence of osteonecrosis of the jaw in patients with oral bisphosphonate exposure. J Oral Maxillofac Surg 68:243–253
Grbic JT, Black DM, Lyles KW, Reid DM, Orwoll E, McClung M, Bucci-Rechtweg C, Su G (2010) The incidence of osteonecrosis of the jaw in patients receiving 5 milligrams of zoledronic acid: data from the health outcomes and reduced incidence with zoledronic acid once yearly clinical trials program. J Am Dent Assoc 141:1365–1370
Black DM, Reid IR, Cauley JA et al (2015) The effect of 6 versus 9 years of zoledronic acid treatment in osteoporosis: a randomized second extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 30:934–944
Cummings SR, Martin JS, McClung MR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Papapoulos S, Chapurlat R, Libanati C et al (2012) Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res 27:694–701
McClung MR, Lewiecki EM, Geller ML, Bolognese MA, Peacock M, Weinstein RL, Ding B, Rockabrand E, Wagman RB, Miller PD (2013) Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int 24:227–235
Papapoulos S, Lippuner K, Roux C et al (2015) The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int 26:2773–2783
Benjamin B, Benjamin MA, Swe M, Sugathan S (2016) Review on the comparison of effectiveness between denosumab and bisphosphonates in post-menopausal osteoporosis. Osteoporos Sarcopenia 2:77–81
Sone T, Kon N, Gaither KW, Okubo N, Osakabe T, Nakayama Y, Fukunaga M, Ito M, Nakamura T (2017) Effects of 3-year denosumab treatment on hip structure in Japanese postmenopausal women and men with osteoporosis. Bone Rep 7:164–171
McClung MR (2017) Denosumab for the treatment of osteoporosis. Osteoporos Sarcopenia 3:8–17
Bone HG, Wagman RB, Brandi ML et al (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5:513–523
Aghaloo TL, Felsenfeld AL, Tetradis S (2010) Osteonecrosis of the jaw in a patient on Denosumab. J Oral Maxillofac Surg 68:959–963
Hanley DA, Adachi JD, Bell A, Brown V (2012) Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract 66:1139–1146
Williams DW, Lee C, Kim T et al (2014) Impaired bone resorption and woven bone formation are associated with development of osteonecrosis of the jaw-like lesions by bisphosphonate and anti-receptor activator of NF-kappaB ligand antibody in mice. Am J Pathol 184:3084–3093
Zebaze RM, Libanati C, Austin M et al (2014) Differing effects of denosumab and alendronate on cortical and trabecular bone. Bone 59:173–179
Ristow O, Gerngross C, Schwaiger M, Hohlweg-Majert B, Kehl V, Jansen H, Hahnefeld L, Koerdt S, Otto S, Pautke C (2014) Effect of antiresorptive drugs on bony turnover in the jaw: denosumab compared with bisphosphonates. Br J Oral Maxillofac Surg 52:308–313
Lipton A, Fizazi K, Stopeck AT et al (2012) Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 48:3082–3092
Qi WX, Tang LN, He AN, Yao Y, Shen Z (2014) Risk of osteonecrosis of the jaw in cancer patients receiving denosumab: a meta-analysis of seven randomized controlled trials. Int J Clin Oncol 19:403–410
Scagliotti GV, Hirsh V, Siena S et al (2012) Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol 7:1823–1829
Henry DH, Costa L, Goldwasser F et al (2011) Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 29:1125–1132
Fizazi K, Carducci M, Smith M et al (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. The Lancet 377:813–822
Huang YF, Chang CT, Muo CH, Tsai CH, Shen YF, Wu CZ (2015) Impact of bisphosphonate-related osteonecrosis of the jaw on osteoporotic patients after dental extraction: a population-based cohort study. PLoS ONE 10:e0120756
Yoneda T, Hagino H, Sugimoto T et al (2017) Antiresorptive agent-related osteonecrosis of the jaw: Position Paper 2017 of the Japanese Allied Committee on Osteonecrosis of the Jaw. J Bone Miner Metab 35:6–19
Hong JW, Nam W, Cha IH, Chung SW, Choi HS, Kim KM, Kim KJ, Rhee Y, Lim SK (2010) Oral bisphosphonate-related osteonecrosis of the jaw: the first report in Asia. Osteoporos Int 21:847–853
Lee JK, Kim KW, Choi JY et al (2013) Bisphosphonates-related osteonecrosis of the jaw in Korea: a preliminary report. J Korean Assoc Oral Maxillofac Surg 39:9–13
Lin TC, Yang CY, Kao Yang YH, Lin SJ (2014) Incidence and risk of osteonecrosis of the jaw among the Taiwan osteoporosis population. Osteoporos Int 25:1503–1511
Chiu WY, Chien JY, Yang WS, Juang JM, Lee JJ, Tsai KS (2014) The risk of osteonecrosis of the jaws in Taiwanese osteoporotic patients treated with oral alendronate or raloxifene. J Clin Endocrinol Metab 99:2729–2735
Tsai MS, Lin MH, Lee CP, Yang YH, Chen WC, Chang GH, Tsai YT, Chen PC, Tsai YH (2017) Chang Gung Research Database: a multi-institutional database consisting of original medical records. Biomed J 40:263–269
Shao SC, Lai EC, Huang TH, Hung MJ, Tsai MS, Yang YH, Chan YY (2021) The Chang Gung Research Database: multi-institutional real-world data source for traditional Chinese medicine in Taiwan. Pharmacoepidemiol Drug Saf 30:652–660
Yarom N, Shapiro CL, Peterson DE et al (2019) Medication-related osteonecrosis of the jaw: MASCC/ISOO/ASCO Clinical Practice Guideline. J Clin Oncol 37:2270–2290
Bone HG, Chapurlat R, Brandi ML et al (2013) The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab 98:4483–4492
Solomon DH, Mercer E, Woo SB, Avorn J, Schneeweiss S, Treister N (2013) Defining the epidemiology of bisphosphonate-associated osteonecrosis of the jaw: prior work and current challenges. Osteoporos Int 24:237–244
Ruggiero SL, Dodson TB, Aghaloo T, Carlson ER, Ward BB, Kademani D (2022) American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J Oral Maxillofac Surg 80:920–943
Hadaya D, Soundia A, Freymiller E, Grogan T, Elashoff D, Tetradis S, Aghaloo TL (2018) Nonsurgical management of medication-related osteonecrosis of the jaws using local wound care. J Oral Maxillofac Surg 76:2332–2339
Watters AL, Hansen HJ, Williams T, Chou JF, Riedel E, Halpern J, Tunick S, Bohle G, Huryn JM, Estilo CL (2013) Intravenous bisphosphonate-related osteonecrosis of the jaw: long-term follow-up of 109 patients. Oral Surg Oral Med Oral Pathol Oral Radiol 115:192–200
Ristow O, Ruckschloss T, Muller M, Berger M, Kargus S, Pautke C, Engel M, Hoffmann J, Freudlsperger C (2019) Is the conservative non-surgical management of medication-related osteonecrosis of the jaw an appropriate treatment option for early stages? A long-term single-center cohort study. J Craniomaxillofac Surg 47:491–499
Carlson ER (2014) Management of antiresorptive osteonecrosis of the jaws with primary surgical resection. J Oral Maxillofac Surg 72:655–657
Williamson RA (2010) Surgical management of bisphosphonate induced osteonecrosis of the jaws. Int J Oral Maxillofac Surg 39:251–255
Wilde F, Heufelder M, Winter K, Hendricks J, Frerich B, Schramm A, Hemprich A (2011) The role of surgical therapy in the management of intravenous bisphosphonates-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 111:153–163
Yuan A, Munz A, Reinert S, Hoefert S (2020) Histologic analysis of medication-related osteonecrosis of the jaw compared with antiresorptive-exposed bone and other infectious, inflammatory, and necrotic jaw diseases. Oral Surg Oral Med Oral Pathol Oral Radiol 129:133–140
Aoki K, Matsunaga S, Ito S, Shibahara T, Nomura T, Matsuzaki H, Abe S, Yamaguchi A (2021) Persistent bone resorption lacunae on necrotic bone distinguish bisphosphonate-related osteonecrosis of jaw from denosumab-related osteonecrosis. J Bone Miner Metab 39:737–747
Chiandussi S, Biasotto M, Dore F, Cavalli F, Cova MA, Di Lenarda R (2006) Clinical and diagnostic imaging of bisphosphonate-associated osteonecrosis of the jaws. Dentomaxillofac Radiol 35:236–243
Pichardo SEC, Broek FWT, Fiocco M, Appelman-Dijkstra NM, van Merkesteyn JPR (2020) A comparison of the cone beam computed tomography findings in medication-related osteonecrosis of the jaws related to denosumab versus bisphosphonates: an observational pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol 129:411–417
Acknowledgements
This study is based in part on data from the Chang Gung Research Database, which was provided by Chang Gung Memorial Hospital. The interpretation and conclusions contained herein do not represent the views of Chang Gung Memorial Hospital. The authors thank the statistical assistance and wish to acknowledge the support of the Maintenance Project of the Center of Data Science and Biostatistics (Grant Nos.: CGRPG2F0011, CLRPG2C0021, CLRPG2C0022, CLRPG2C0023, CLRPG2C0024, CLRPG2G0081, CLRPG2G0082, CLRPG2G0083, CLRPG2L0021, and CLRPG2L0022) at Keelung Chang Gung Memorial Hospital for assistance with the study design and monitoring, data analysis, and interpretation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Liu, FC., Luk, KC. & Chen, YC. Risk comparison of osteonecrosis of the jaw in osteoporotic patients treated with bisphosphonates vs. denosumab: a multi-institutional retrospective cohort study in Taiwan. Osteoporos Int 34, 1729–1737 (2023). https://doi.org/10.1007/s00198-023-06818-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06818-3