Abstract
Summary
In postmenopausal women with osteoporosis, up to 10 years of denosumab treatment significantly and continuously improved bone microarchitecture assessed by tissue thickness–adjusted trabecular bone score, independently of bone mineral density. Long-term denosumab treatment decreased the number of high fracture-risk patients and shifted more patients to lower fracture-risk categories.
Purpose
To investigate the long-term effect of denosumab on bone microarchitecture assessed by tissue thickness–adjusted trabecular bone score (TBSTT) in post-hoc subgroup analysis of FREEDOM and open-label extension (OLE).
Methods
Postmenopausal women with lumbar spine (LS) or total hip BMD T-score <−2.5 and ≥−4.0 who completed the FREEDOM DXA substudy and continued in OLE were included. Patients received either denosumab 60 mg subcutaneously every 6 months for 3 years and same-dose open-label denosumab for 7 years (long-term denosumab; n=150) or placebo for 3 years and open-label denosumab for 7 years (crossover denosumab; n=129). BMD and TBSTT were assessed on LS DXA scans at FREEDOM baseline, month 1, and years 1–6, 8, and 10.
Results
In long-term denosumab group, continued increases from baseline to years 4, 5, 6, 8, and 10 in BMD (11.6%, 13.7%, 15.5%, 18.5%, and 22.4%) and TBSTT (3.2%, 2.9%, 4.1%, 3.6%, and 4.7%) were observed (all P < 0.0001). Long-term denosumab treatment decreased the proportion of patients at high fracture-risk (according to TBSTT and BMD T-score) from baseline up to year 10 (93.7 to 40.4%), resulting in increases in the proportions at medium-risk (6.3 to 53.9%) and low-risk (0 to 5.7%) (P < 0.0001). Similar responses were observed in crossover denosumab group. Changes in BMD and TBSTT were poorly correlated during denosumab treatment.
Conclusion
In postmenopausal women with osteoporosis, up to 10 years of denosumab significantly and continuously improved bone microarchitecture assessed by TBSTT, independently of BMD, and shifted more patients to lower fracture-risk categories.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to bone fragility and a consequent increase in fracture risk [1]. Bone mass is measured by bone mineral density (BMD) using dual-energy X-ray absorptiometry (DXA). BMD is a major determinant of bone strength and the gold standard for the diagnosis of osteoporosis [2]. Decreased BMD is considered to be an important predictor of osteoporotic fractures [3]. However, the risk of fracture also depends on factors other than BMD, since many individuals with a fragility fracture have non-osteoporotic BMD values [4, 5].
Evaluating bone microarchitecture, indirectly measured by trabecular bone score (TBS), enhances the assessment of bone strength and fracture risk, beyond BMD assessment alone [2]. TBS is a gray-level textural metric extracted from DXA scans; it uses 2-dimensional DXA images to characterize the variations in gray-level amplitude in the corresponding 3-dimensional tissue microarchitecture [6, 7]. A high TBS value correlates with a large number of low-amplitude variations that indicate more homogeneous and stronger bone microstructure, while a low TBS value correlates with a low number of high-amplitude variations that indicate more variable, separated, and deteriorated bone microstructure [6, 8]. TBS is predictive of osteoporotic fractures independently of BMD and/or clinical risk factors from the Fracture Risk Assessment Tool (FRAX®) and has been shown to complement both BMD and FRAX® to improve fracture risk prediction [2, 6, 9–14]. TBS has been included in many national and international guidelines such as the International Society for Clinical Densitometry (ISCD) Official Positions [15] and American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE) Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis [16] as a readily available, noninvasive tool beyond standard BMD measurements and clinical risk factors to improve categorization of patients at risk for fracture [2].
The TBS algorithm accounts for the presence of regional soft tissues, as the gray-level texture of the DXA image depends on both the bone and soft tissues, with the latter attenuating the X-rays, gray level variations, and eventually the TBS value [17]. The recently updated TBS algorithm directly corrects for the soft tissue thickness (tissue thickness–adjusted TBS or TBSTT) in the region of the spine, replacing the previously used TBS algorithm correcting for body mass index (BMI), a proxy for soft tissue thickness. It has been shown that TBSTT neutralizes the regional soft tissue noise on DXA images better than BMI-adjusted TBS (TBSBMI) as it is less dependent on whole body morphotype [18].
In postmenopausal women, increased tissue exposure to RANK ligand due to estrogen deficiency accelerates bone resorption and induces bone loss, leading to osteoporosis [19]. Denosumab is a fully human monoclonal antibody against RANK ligand that reduces osteoclast number and activity and decreases bone resorption [20–23]. During the 3-year phase 3 FREEDOM study in postmenopausal women with osteoporosis, denosumab treatment significantly increased BMD and reduced the risk of vertebral, nonvertebral, and hip fracture compared with placebo [24]. A subset of women participated in a prospective FREEDOM DXA substudy, in which they underwent more extensive BMD assessments and showed significant BMD gains comparable to those in the overall FREEDOM population [25]. Because of the chronic nature of osteoporosis, the 3-year FREEDOM trial was followed by a 7-year open-label extension (OLE), during which all patients received denosumab. Up to 10 years of denosumab treatment was associated with continued increases in BMD and low incidence of new vertebral and nonvertebral fractures [26, 27].
To explore the effect of long-term denosumab treatment on TBS and bone microarchitecture, this FREEDOM TBS post-hoc analysis employed the updated TBS algorithm (TBSTT) [18] to evaluate lumbar spine (LS) DXA scans of women who completed the DXA substudy and enrolled in the OLE study. The initial report based on the 3-year DXA substudy demonstrated that denosumab significantly improved TBSTT vs placebo independently of BMD [28]. Further, TBSTT showed greater changes from baseline and larger differences from placebo than TBSBMI in response to denosumab treatment [28]. Here, we present the treatment outcome of long-term denosumab on TBSTT and bone microarchitecture during the OLE with denosumab administration for up to 7 or 10 years.
Methods
Study design and patients
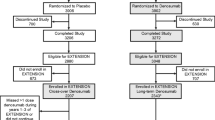
Study designs of the FREEDOM (NCT00089791) and OLE (NCT00523341) studies and the DXA substudy have previously been described [24–26] (Fig. 1). FREEDOM was a phase 3, multicenter, randomized, double-blind, placebo-controlled study conducted at 213 centers worldwide in 7808 postmenopausal women with a BMD T-score between −4.0 and −2.5 at the lumbar spine or total hip. Patients were randomized 1:1 centrally to receive either denosumab 60 mg or placebo subcutaneously every 6 months for 3 years and were instructed to take daily supplementation of calcium (≥1000 mg) and vitamin D (≥400 IU). All patients who completed the FREEDOM study without discontinuing treatment or missing more than one dose of study drug were eligible to enter the OLE and receive open-label denosumab 60 mg subcutaneously every 6 months for 7 years. The prospective DXA substudy was conducted at 19 centers that performed more frequent BMD assessments and evaluations of additional skeletal sites for 441 women. Informed consent was obtained for all patients in the FREEDOM and OLE studies and the DXA substudy.
This FREEDOM TBS post-hoc analysis analyzed TBSTT on 2409 LS DXA scans collected from 279 women who completed the DXA substudy and rolled over to the OLE study (Fig. 1). Patients with BMI >38 kg/m2 or <15 kg/m2 were excluded as it was out of the manufacturer-recommended range for a proper assessment of TBS. Similarly, patients were excluded if the DXA scanner or the acquisition mode used for BMD assessment was not compatible with TBS algorithm. Women who received 3 years of denosumab in the DXA substudy and continued in the OLE could have up to 10 years of denosumab exposure (long-term denosumab group). Women who received 3 years of placebo in the DXA substudy and crossed over to denosumab in the OLE could have up to 7 years of denosumab exposure (crossover denosumab group).
Study assessments
LS (L1-L4) DXA scans were performed using Lunar (GE Healthcare, Madison, WI, USA) or Hologic (Hologic Inc., Bedford, MA, USA) DXA bone densitometers at FREEDOM baseline, month 1, and years 1, 2, and 3 in the DXA substudy [25] and at OLE baseline and OLE years 1, 2, 3, 5, and 7. For an individual patient, the same type of machine was used for all measurements throughout the study. All DXA scans were centrally read by Clario (formerly BioClinica or Synarc; Princeton, NJ, USA) [25].
TBSTT was assessed using a pre-release of TBS iNsight software version 4.0 (Medimaps group, Geneva, Switzerland) to compensate for the soft tissue thickness of the specific region of interest directly. TBSTT was calculated as the mean of the individual measurements for each included vertebra. The calculation was performed blinded from clinical outcomes and treatment group allocation. Both LS BMD and TBSTT were calculated from the LS DXA scans in the same region of interest (L1-L4) [28]. Fractured vertebrae (prevalent or incident), as confirmed by X-rays, or clearly abnormal and non-assessable vertebrae were excluded from the assessments of both BMD and TBSTT, following the ISCD criteria [15].
Vertebral osteoporotic fractures were centrally identified by Clario based on lateral spine radiographs by a semiquantitative grading scale [29]. Clinical and nonvertebral osteoporotic fractures required confirmation by diagnostic imaging or a radiologist’s report [24, 26].
Statistical analyses
This analysis included patients who completed the DXA substudy and continued to the OLE and had LS BMD and TBSTT measurements at baseline and at least one post-baseline visit. Missing data were not imputed. The percent changes in LS BMD and TBSTT from baseline to every year up to year 10 were analyzed using a repeated-measures mixed effects linear model, adjusting for visit, baseline value, machine type, treatment-by-visit interaction, and baseline value-by-machine type interaction. Statistical inferences on (i) differences between the denosumab and placebo groups during the 3-year DXA substudy, (ii) within-group differences from FREEDOM baseline for the placebo group and the long-term denosumab group over up to 10 years, and (iii) within-group differences from OLE baseline for the crossover denosumab group over up to 7 years (Fig. 1) were assessed at appropriate follow-up timepoints with no adjustment for multiplicity. The results were reported as least-squares means and associated two-sided 95% confidence intervals (CIs).
Bone microarchitecture based on TBSTT fracture risk categories (TBSTT ≤1.027, degraded microarchitecture with high fracture risk; TBSTT >1.027 to ≤1.074, partially degraded microarchitecture with medium fracture risk; TBSTT >1.074, normal microarchitecture with low fracture risk) was evaluated at baseline and up to year 3 for the placebo group, at OLE baseline and up to OLE year 7 for the crossover denosumab group, and at baseline and up to year 10 for the long-term denosumab group. These TBSTT risk thresholds of 1.027 and 1.074 are equivalent to 1.230 and 1.310, the risk thresholds reported for the classical TBSBMI algorithm, because TBSTT thresholds were derived from the same analysis of the same population that generated TBSBMI thresholds [12]. At the commercial release of the newest version of TBSTT, the thresholds will be calibrated to historical ones for clinical continuity. Statistical inferences on the significance of the within-group change in the percentage of patients in each TBSTT degradation category from baseline to the respective follow-up timepoint were evaluated using Bhapkar’s test for homogeneity.
The effect of long-term denosumab treatment on the risk of osteoporotic fractures was analyzed by TBSTT and BMD T-score risk categories (BMD T-score ≤−2.5, or TBSTT ≤1.027 and −2.5 < BMD <−1.0, high risk; TBSTT >1.027 and −2.5 < BMD <−1.0, or BMD ≥−1.0 and TBSTT ≤ 1.074, medium risk; BMD ≥−1.0 and TBSTT >1.074, low risk). The percentages of patients in each risk category in the crossover denosumab group at OLE baseline and up to OLE year 7 and in the long-term denosumab group at baseline and up to year 10 were summarized, and statistical inferences on the significance of the changes were evaluated using Bhapkar’s test for homogeneity. To further assess the association between change in TBSTT and fracture risk, the incidence of vertebral fractures, nonvertebral fractures, or clinical fractures was calculated for patients stratified into tertiles of mean TBSTT percentage change from OLE baseline until up to OLE year 7 in the crossover denosumab group and from FREEDOM baseline until up to year 10 in the long-term denosumab group.
The correlation between LS BMD and TBSTT was evaluated using Pearson correlation coefficients for mean percentage changes from baseline to last follow-up timepoints within the placebo group, the crossover denosumab group, and the long-term denosumab group.
Results
This analysis included a total of 279 postmenopausal women with osteoporosis: 150 women received up to 10 years of denosumab treatment during the entire study; 129 women received placebo for 3 years during the DXA substudy and then crossed over to receive up to 7 years of denosumab treatment during the OLE (Fig. 1). Baseline characteristics were mostly balanced between the placebo group and the long-term denosumab group at FREEDOM baseline. The mean LS BMD T-score was −2.8, and mean TBSTT was 1.03 in all patients included in the analysis at FREEDOM baseline. About 19% and 25% of women had a vertebral fracture in the placebo and the long-term denosumab groups, respectively, at FREEDOM baseline. At OLE baseline, patients in the long-term denosumab group, who had received 3 years of denosumab before entering the OLE, had higher mean LS BMD T-score (−2.1) and TBSTT (1.06) than patients in the crossover denosumab group (−2.8, and 1.02, respectively) (Table 1).
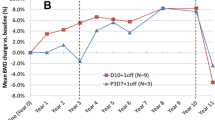
Long-term treatment with denosumab was associated with continued increases in LS BMD and TBSTT during the OLE study (Fig. 2). Mean percentage changes from FREEDOM baseline to years 4, 5, 6, 8, and 10 in LS BMD (least-squares mean increases of 11.6%, 13.7%, 15.5%, 18.5%, and 22.4%, respectively) and TBSTT (3.2%, 2.9%, 4.1%, 3.6%, and 4.7%, respectively) in the long-term denosumab group were all statistically significant (all P < 0.0001). Mean percentage changes from OLE baseline to OLE years 1, 2, 3, 5, and 7 in LS BMD (least-squares mean increases of 5.3%, 7.7%, 9.6%, 13.0%, and 17.2%, respectively; all P < 0.0001) and TBSTT (0.3%, 0.9%, 1.1% [P < 0.05], 2.2%, and 3.2% [P < 0.0001], respectively) were also increasing in the crossover denosumab group. The overall trend of improvement in LS BMD and TBSTT observed in the crossover denosumab group from OLE baseline to OLE year 7 largely replicated that observed in the long-term denosumab group during the first 7 years of denosumab treatment (Fig. 2).
Percentage change from baseline by visit and treatment group for (A) LS BMD and (B) TBSTT. Data are presented as least-squares mean and 95% confidence interval. n = number of patients with observed data. *P < 0.05 compared with OLE baseline; **P < 0.0001 compared with baseline; ***P < 0.0001 compared with OLE baseline; †P < 0.001 compared with placebo; ‡P < 0.0001 compared with placebo. BL, baseline; BMD, bone mineral density; LS, lumbar spine; TBS, trabecular bone score; TBSTT, tissue thickness–adjusted TBS
Long-term denosumab therapy led to more patients having normal microarchitecture, as defined by TBSTT, and fewer patients with degraded or partially degraded microarchitecture (Fig. 3). In the long-term denosumab group, the number of patients with normal microarchitecture increased from 26.1% at baseline to 53.2% up to year 10, and the number of patients with degraded or partially degraded microarchitecture decreased from 48.6 to 29.1% and from 25.4 to 17.7%, respectively (P < 0.0001). A similar improvement in bone microstructure was observed in the crossover denosumab group from OLE baseline over up to 7 years of denosumab treatment (P < 0.0001). In comparison, bone microstructure remained unchanged or slightly deteriorated in the placebo group from FREEDOM baseline to year 3 (P = 0.0480).
Percentage of patients by TBSTT risk category in the placebo group at baseline and year 3, in the crossover denosumab group at OLE baseline and up to OLE year 7, and in the long-term denosumab group at baseline and up to year 10. n = number of patients with observed data. P value based on Bhapkar’s test for homogeneity. TBS, trabecular bone score; TBSTT, tissue thickness–adjusted TBS. Degraded, TBSTT ≤1.027; partially degraded, TBSTT >1.027 to ≤1.074; normal, TBSTT >1.074
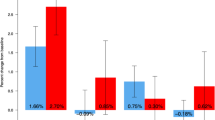
When the risk of osteoporotic fracture was assessed based on both TBSTT and BMD T-score, long-term denosumab therapy was found to result in more patients exiting the high-risk category and entering the medium- and low-risk categories (Fig. 4a). In the long-term denosumab group, the number of patients in the high-risk category decreased from 93.7% at baseline to 40.4% at up to year 10, and in the medium-risk and low-risk categories increased from 6.3 to 53.9% and from 0 to 5.7%, respectively (P < 0.0001). A similar effect of treatment shifting patients into lower risk categories was observed in the crossover denosumab group from OLE baseline to up to OLE year 7 (high risk, 89.6 to 48.7%; medium risk, 10.4 to 49.6%; and low risk, 0 to 1.7%; P < 0.0001).
Fracture risk by TBSTT and BMD T-score (A) or tertiles of mean TBSTT percent change (B). BMD, bone mineral density. TBS, trabecular bone score; TBSTT, tissue thickness–adjusted TBS. A Percentage of patients within each TBSTT and BMD T-score risk category in the crossover denosumab group at OLE baseline and up to OLE year 7 and in the long-term denosumab group at baseline and up to year 10. n = number of patients with observed data. P value based on Bhapkar’s test for homogeneity. High risk, BMD T-score ≤−2.5, or TBSTT ≤1.027 and −2.5 < BMD <−1.0; medium risk, TBSTT >1.027 and −2.5 < BMD < −1.0, or BMD ≥−1.0 and TBSTT ≤ 1.074; low risk, BMD ≥−1.0 and TBSTT >1.074. B Incidence of new or worsening vertebral fractures, nonvertebral fractures, and clinical fractures by tertiles of mean TBSTT percentage change in the crossover denosumab group from OLE baseline until up to OLE year 7 and in the long-term denosumab group from baseline until up to year 10. n = number of patients with observed TBSTT data at baseline and the specified timepoint within each tertile. Patients within the lowest tertile had the smallest mean TBSTT percentage change, and patients within the highest tertile had the largest mean TBSTT percentage change
Consistent with TBS as a predictor of osteoporotic fractures [2, 6, 9–14], patients who achieved TBSTT improvement in the highest tertile (i.e., the largest TBSTT improvement) during long-term denosumab treatment tended to have reduced fracture risk (Fig. 4b). Only a small group of patients who had TBSTT data at both baseline and year 10 (i.e., OLE year 7) were analyzed for fracture incidences by tertiles. In the long-term denosumab group (n=66), incidences of new or worsening vertebral fractures, nonvertebral fractures, and clinical fractures occurring up to year 10 were 4.8%, 0%, and 0%, respectively, in patients with the largest TBSTT percentage change from baseline to up to year 10, compared to 13.6%, 4.5%, and 9.1%, respectively, in patients with smallest TBSTT changes, and 17.4%, 8.7%, and 13.0%, respectively, in patients with medium TBSTT changes. This trend was also observed in the crossover denosumab group (n=56) when comparing patients with the largest TBSTT percentage change from OLE baseline to up to OLE year 7 (vertebral, nonvertebral, and clinical fractures occurring up to OLE year 7, 0%, 0%, and 0%, respectively) versus patients with smallest TBSTT changes (10.5%, 10.5%, and 10.5%, respectively) and medium TBSTT changes (5.3%, 21.1%, and 26.3%, respectively).
Over the course of long-term denosumab treatment, changes in TBSTT were largely unrelated to changes in LS BMD (Fig. 5). Pearson correlation coefficient between the mean percentage changes of TBSTT and LS BMD was 0.05 from baseline to year 10 in the long-term denosumab group and 0.28 from OLE baseline to OLE year 7 in the crossover denosumab group, both of which were below the threshold commonly interpreted as poor correlation (<0.3) [30, 31].
Relationship between LS BMD percentage change and TBSTT percentage change in the placebo group from baseline to year 3, in the crossover denosumab group from OLE baseline to OLE year 7, and in the long-term denosumab group from baseline to year 10. n = number of patients with observed BMD and TBSTT data at the specified timepoint. Pearson correlation between the mean percentage changes of BMD and TBSTT: r2 = 0.079 in the placebo group from baseline to year 3; r2 = 0.278 in the crossover denosumab group from OLE baseline to OLE year 7; r2 = 0.050 in the long-term denosumab group from baseline to year 10. BMD, bone mineral density; LS, lumbar spine; TBS, trabecular bone score; TBSTT, tissue thickness–adjusted TBS
Discussion
Results from this FREEDOM TBS post-hoc analysis showed that up to 10 years of denosumab treatment significantly and continuously improved TBSTT and bone microarchitecture in postmenopausal women with osteoporosis. The correlation between changes in TBSTT and LS BMD remained poor over 10 years, confirming that the two measures provide independent information on bone strength. Long-term denosumab shifted more patients to lower-risk categories assessed by both TBSTT and BMD T-score. Patients with the largest TBSTT improvement during long-term denosumab treatment tended to experience a reduced incidence of fractures, supporting the importance of improving TBS in patients receiving anti-osteoporotic therapies.
TBS, a noninvasive measurement of bone microarchitecture based upon DXA images, is sensitive to changes over time from either natural disease progression or osteoporosis treatment. Different therapies have been shown to impact TBS to different degrees [7, 13, 14], likely due to their differential effects on the two components of bone remodeling: resorption and formation. For antiresorptive agents, the magnitude of changes in TBS was greater with denosumab treatment compared with bisphosphonates (e.g., alendronate, risedronate, ibandronate, zoledronic acid) [13, 32, 33]. For the bone-building agents, teriparatide and romosozumab, TBS changes were faster and of greater amplitude than antiresorptive agents [34]. However, previous studies assessing the effect of bone-affecting therapies on TBS were conducted for no more than 3 years.
The current analysis is the first report to evaluate the long-term impact of denosumab treatment for up to 10 years on bone microstructure as assessed by the updated TBSTT computation algorithm, which directly corrects for the effect of regional soft tissue thickness on the DXA image and has been clinically validated to evaluate TBS and maintain its clinical performance [18]. Our results show that the initial increase in TBSTT observed during the 3-year FREEDOM DXA substudy [28] persisted with long-term denosumab treatment in the OLE, with bone microstructure showing consistent improvement over time. Denosumab has been shown previously to prevent plate perforation and preserve axially aligned trabeculae [35], and modeling-based bone formation could occur despite the potent inhibitory effect of denosumab on bone resorption [36], all of which may have contributed to the observed long-term improvement in trabecular bone microstructure. This first observation of continued improvements in TBSTT and bone microstructure for up to 10 years with an osteoporosis therapy, denosumab, is important as it may lead to a better understanding of the role of TBS in monitoring therapeutic responses and guiding management of patients undergoing long-term treatment.
Previous studies have shown that TBS provides a measure of bone strength not captured by BMD [7]. Our results confirmed that even with up to 10 years of treatment, changes in TBSTT and changes in LS BMD were largely unrelated, supporting the independent and complementary nature of the two measurements. TBS can predict osteoporotic fractures as well as BMD in postmenopausal women [7] and improve fracture risk prediction when used in conjunction with BMD [2, 9–13]. Our results provide further support and show that patients who achieved the largest TBSTT changes during long-term denosumab treatment tended to have reduced incidences of new or worsening vertebral fractures, nonvertebral fractures, and clinical fractures, compared to patients with smaller TBSTT changes. It is worth noting that this analysis was descriptive in nature, and the small sample size within each tertile might be associated with high variance of fracture incidences, which may explain why patients with medium TBSTT changes, unexpectedly, had slightly higher incidences of fractures than patients with the smallest TBSTT changes. However, the overall trend of reduced fracture incidences in patients with the largest TBSTT changes supports a role for TBS as a predictor of osteoporotic fractures [7]. When fracture risk was evaluated by both TBSTT and BMD T-score in all patients who completed the DXA substudy and rolled over into the OLE study, we found that long-term denosumab reduced the number of patients at high risk, shifting a majority into lower risk categories. This result is consistent with the maintenance of low fracture incidence over 10 years of therapy with denosumab in the overall population of FREEDOM and OLE [26].
Limitations of the present analysis include the absence of a placebo control over the long-term denosumab treatment period and the lack of direct measures of trabecular architecture. However, placebo could not be continued beyond 3 years in the OLE in consideration of the well-being of the patients. In addition, the crossover denosumab group showed very similar results compared with the long-term denosumab group, confirming the treatment effect of long-term denosumab therapy. Although TBS is an indirect measure of trabecular microarchitecture, it has been correlated with bone microarchitecture measures such as connectivity density, trabecular number, trabecular separation, trabecular bone volume over tissue volume, and structure model index in several studies, and is a US Food and Drug Administration–approved application to DXA images [2, 6, 37]. DXA scanners involved in the study were not TBS calibrated using a fractal dedicated phantom. TBSTT categories may have smaller within group variability if calibration phantom was applied. Although long-term treatment with denosumab led to significant increases in TBSTT, 4.7% on average in a group of 66 patients at year 10 (Fig. 2b), one should be cautious in interpreting TBSTT changes from baseline at the individual level without considering the least significant change of both BMD and TBS at your site. This study is a retrospective analysis in a small subset of the total FREEDOM population, which may limit the generalizability of the study results. Nevertheless, the effect of long-term denosumab on TBSTT likely reflects the effect in the originally randomized patients in FREEDOM because the baseline characteristics and LS BMD changes over up to 10 years of denosumab treatment in this FREEDOM TBS post-hoc analysis were similar to those reported in the overall population [24, 26].
In conclusion, long-term denosumab resulted in continued increases in TBSTT and improved bone microarchitecture in postmenopausal women with osteoporosis. This treatment effect was observed over a longer duration of treatment (up to 10 years) than in previous osteoporosis trials [13, 33] and detected using the updated TBSTT algorithm that better adjusts for regional soft tissue thickness [18]. Changes in TBSTT were poorly correlated with changes in LS BMD over up to 10 years of denosumab treatment, supporting the independent and complementary role of TBSTT to BMD. Long-term denosumab treatment shifted more patients to lower fracture-risk categories based on both TBSTT and BMD T-score, and patients with the largest TBSTT improvement trended toward having a reduced incidence of fracture. Different osteoporosis therapies have differential effects on resorption and formation of bone remodeling and may vary in their efficacies and time frames to impact bone structure and/or density. TBS, a convenient tool for evaluating bone microarchitecture and predicting fracture risk, should be incorporated into clinical practice, along with BMD and other risk factors, for monitoring treatment responses and managing patients undergoing long-term osteoporosis therapy.
Change history
04 March 2023
Graphical Abstract was replaced with high resolution file.
References
Schuit SC, van der Klift M, Weel AE et al (2004) Fracture incidence and association with bone mineral density in elderly. Bone 34:195–202
Silva BC, Broy SB, Boutroy S, Schousboe JT, Shepherd JA, Leslie WD (2015) Fracture risk prediction by non-BMD DXA measures: the 2015 ISCD Official Positions Part 2: trabecular bone score. J Clin Densitom 18:309–330
McClung MR (2005) The relationship between bone mineral density and fracture risk. Curr Osteoporos Rep 3:57–63
Siris ES, Chen YT, Abbott TA, Barrett-Connor E, Miller PD, Wehren LE, Berger ML (2004) Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 164:1108–1112
Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD (2007) Low bone mineral density and fracture burden in postmenopausal women. CMAJ 177:575–580
Hans D, Barthe N, Boutrop S et al (2011) Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom 14:302–312
Silva BC, Leslie WD, Resch H, Lamy O, Lesnyak O, Binkley N et al (2014) Trabecular bone score: a noninvasive analytical method based upon the DXA image. J Bone Miner Res 29:518–530
Muschitz C, Kocijan R, Haschka J et al (2015) TBS reflects trabecular microarchitecture in premenopausal women and men with idiopathic osteoporosis and low-traumatic fractures. Bone 79:259–266
Winzenrieth R, Dufour R, Pothuaud L, Hans D (2010) A retrospective case-control study assessing the role of trabecular bone score in postmenopausal Caucasian women with osteopenia: analyzing the odds of vertebral fracture. Calcif Tissue Int 86:104–109
McCloskey EV, Odén A, Harvey NC et al (2015) Adjusting fracture probability by trabecular bone score. Calcif Tissue Int 96:500–509
Leslie WD, Johansson H, Kanis JA, Lamy O, Oden A, McCloskey EV, Hans D (2014) Lumbar spine texture enhances 10-year fracture probability assessment. Osteoporos Int 25:2271–2277
McCloskey EV, Odén A, Harvey NC, Leslie WD, Hans D et al (2016) A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res 31:940–948
Hans D, Šteňová E, Lamy O (2017) The trabecular bone score (TBS) complements DXA and the FRAX as a fracture risk assessment tool in routine clinical practice. Curr Osteoporos Rep 15:521–531
Shevroja E, Lamy O, Kohlmeier L, Koromani F, Rivadeneira F, Hans D (2017) Use of trabecular bone score (TBS) as a complementary approach to dual-energy X-ray absorptiometry (DXA) for fracture risk assessment in clinical practice. J Clin Densitom 20:334–345
Shuhart CR, Yeap SS, Anderson PA et al (2019) Executive summary of the 2019 ISCD position development conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, peri-prosthetic and orthopedic bone health, transgender medicine, and pediatrics. J Clin Densitom 22:453–471
Camacho PM, Petak SM, Binkley N et al (2020) American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2020 update. Endocr Pract 26(Suppl 1):1–46
Amnuaywattakorn S, Sritara C, Utamakul C et al (2016) Simulated increased soft tissue thickness artefactually decreases trabecular bone score: a phantom study. BMC Musculoskelet Disord 17:17
Shevroja E, Aubry-Rozier B, Hans G, Gonzalez- Rodriguez E, Stoll D, Lamy O, Hans D (2019) Clinical performance of the updated trabecular bone score (TBS) algorithm, which accounts for the soft tissue thickness: the OsteoLaus study. J Bone Miner Res 34:2229–2237
Hofbauer LC, Schoppet M (2004) Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 292:490–495
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T et al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M et al (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95:3597–3602
Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, Rattan A et al (2000) Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 157:435–448
Udagawa N, Takahashi N, Yasuda H, Mizuno A, Itoh K, Ueno Y et al (2000) Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 141:3478–3484
Cummings SR, San Martin J, McClung MR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Bolognese MA, Teglbjærg CS, Zanchetta JR et al (2013) Denosumab significantly increases DXA BMD at both trabecular and cortical sites: results from the FREEDOM study. J Clin Densitom 16:147–153
Bone HG, Wagman RB, Brandi ML et al (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5:513–523
Ferrari S, Butler PW, Kendler DL, Miller PD, Roux C, Wang AT, Huang S, Wagman RB, Lewiecki EM (2019) Further nonvertebral fracture reduction beyond 3 years for up to 10 years of denosumab treatment. J Clin Endocrinol Metab 104:3450–3461
Hans D, Shevroja E, McDermott M et al (2022) Updated trabecular bone score accounting for the soft tissue thickness (TBSTT) demonstrated significantly improved bone microstructure with denosumab in the FREEDOM TBS post-hoc analysis. Osteoporos Int 33:2517–2525
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Akoglu H (2018) User’s guide to correlation coefficients. Turk J Emerg Med 18:91–93
Rovetta A (2020) Raiders of the lost correlation: a guide on using Pearson and Spearman coefficients to detect hidden correlations in medical sciences. Cureus 12:e11794
McClung MR, Lippuner K, Brandi ML, Zanchetta JR, Bone HG, Chapurlat R, Hans D, Wang A, Zapalowski C, Libanati C (2017) Effect of denosumab on trabecular bone score in postmenopausal women with osteoporosis. Osteoporos Int 28:2967–2973
Di Gregorio S, Del Rio L, Rodriguez-Tolra J, Bonel E, García M, Winzenrieth R (2015) Comparison between different bone treatments on areal bone mineral density (aBMD) and bone microarchitectural texture as assessed by the trabecular bone score (TBS). Bone 75:138–143
Jeong C, Kim J, Lim Y et al (2021) Effect of romosozumab on trabecular bone score compared to anti-resorptive agents in postmenopausal women with osteoporosis. J Bone Metab 28:317–323
Hu YJ, Chines A, Shi Y et al (2022) The effect of denosumab and alendronate on trabecular plate and rod microstructure at the distal tibia and radius: a post-hoc HR-pQCT study. Bone 154:116187
Dempster DW, Chines A, Bostrom MP et al (2020) Modeling-based bone formation in the human femoral neck in subjects treated with denosumab. J Bone Miner Res 35:1282–1288
Roux JP, Wegrzyn J, Boutroy S et al (2013) The predictive value of trabecular bone score (TBS) on whole lumbar vertebrae mechanics: an ex vivo study. Osteoporos Int 24:2455–2460
Acknowledgements
T Jinling Wu (BioScience Communications, New York, NY) provided writing and editorial support.
Data availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request/.
Funding
Open access funding provided by University of Lausanne. This study was funded by Amgen Inc., Thousand Oaks, CA, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study complied with the principles of the Declaration of Helsinki. Institutional review boards and ethics committees approved the protocol and consent process. All procedures performed in studies involving humans were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual patients included in the study.
Conflicts of interest
Didier Hans is the co-owner of the TBS patent and has corresponding ownership shares and position at Medimaps group. Enisa Shevroja has no conflict of interest. Michael McClung received honorarium and consulting fees from Amgen. Michele McDermott is an employee and stockholder of Amgen. Shuang Huang is an employee and stockholder of Amgen. Min Kim is an employee and stockholder of Amgen.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hans, D., McDermott, M., Huang, S. et al. Long-term effect of denosumab on bone microarchitecture as assessed by tissue thickness–adjusted trabecular bone score in postmenopausal women with osteoporosis: results from FREEDOM and its open-label extension. Osteoporos Int 34, 1075–1084 (2023). https://doi.org/10.1007/s00198-023-06708-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06708-8