Abstract
The aim of this systematic review and meta-analysis was (1) to determine exercise effects on bone mineral density (BMD) in postmenopausal women and (2) to address the corresponding implication of bone and menopausal status or supervision in postmenopausal women. A comprehensive search of eight electronic databases according to the PRISMA statement up to August 9, 2022, included controlled exercise trials ≥ 6 months. BMD changes (standardized mean differences: SMD) at the lumbar spine (LS), femoral neck (FN), and total hip (TH) were considered as outcomes. Study group comparisons were conducted for osteopenia/osteoporosis versus normal BMD, early versus late postmenopausal women, and predominantly supervised versus predominantly non-supervised study arms. We applied an inverse heterogeneity (IVhet) model. In summary, 80 studies involving 94 training and 80 control groups with a pooled number of 5581 participants were eligible. The IVhet model determined SMDs of 0.29 (95% CI: 0.16–0.42), 0.27 (95% CI: 0.16–0.39), and 0.41 (95% CI: 0.30–0.52) for LS, FN, and THBMD, respectively. Heterogeneity between the trial results varied from low (I2 = 20%, TH BMD) to substantial (I2 = 68%, LS-BMD). Evidence for publication bias/small study effects was negligibly low (FN-, TH-BMD) to high (LSBMD). We observed no significant differences (p > .09) for exercise effects on LS-, FN-, or TH-BMD-LS between studies/study arms with or without osteopenia/osteoporosis, early versus late postmenopausal women, or predominantly supervised versus non-supervised exercise programs. Using robust statistical methods, the present work provides further evidence for a positive effect of exercise on BMD in postmenopausal women. Differences in bone status (osteopenia/osteoporosis versus normal bone), menopausal status (early versus late postmenopausal), and supervision (yes versus no) did not significantly affect the exercise effects on BMD at LS or proximal femur.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exercise is a promising agent for preventing osteoporosis in postmenopausal women. In their recent comprehensive systematic review and meta-analysis with 75 eligible studies, Shojaa et al. [1] provided definite evidence for the favorable effect of exercise on bone mineral density (BMD). Nevertheless, effect sizes in the predominantly healthy cohorts for lumbar spine (LS) and femoral neck (FN)-BMD were moderate at best. However, the substantial heterogeneity between the trials indicates that some studies were much more effective in improving BMD at LS or FN than others. Several factors might contribute to this aspect. Two factors, bone and menopausal status, might particularly contribute to the high degree of heterogeneity between the trials. While the early-postmenopausal stage is related to increased bone turnover with negative net effects on BMD in many women [2, 3], a factor that might dilute the exercise-induced effect on BMD, there is some evidence for more favorable exercise effects in people with osteopenia/osteoporosis compared to people with normal BMD [4]. However, the most striking effect on heterogeneity of exercise trial findings within comprehensive meta-analysis is differences in the exercise protocols. In this context, supervision of the exercise protocol has far-reaching consequences on setting, exercise composition, feasibility, safety, motivation, and adherence [5, 6]. In a recent systematic review and meta-analysis, we clearly demonstrated the favorable effect of supervised exercise protocols on fracture incidence [7]. Considering the higher complexity of exercise protocols for improving BMD compared to decreasing the number of falls [8], the role of supervision might be even more important in the area of bone strengthening.
In the present comprehensive systematic review and meta-analysis, we thus aimed to (a) provide a 2022 update regarding the effect of exercise on BMD at the LS, FN, and total hip (TH) regions of interest (ROI) using the inverse heterogeneity model (IVhet, [9]) that is less susceptible to underestimation of statistical error in heterogeneous studies and (b) to determine the relevance of the potentially confounding effect of bone status, menopausal status, and supervision of the session on exercise effects on BMD at LS, FN, and TH.
Methods
Data sources and search strategy
Electronic literature searches were conducted using PubMed, Web of Science, Cochrane, Science Direct, Eric, and ProQuest databases up to August 09, 2022, without any language restriction. The keywords and MeSH terms used in the search strategy included (“Bone” or “Bone mass” or “Bone status” or “Bone structure” or “Bone turnover” or “Bone metabolism” or “Bone mineral content” or “Skeleton” or “Bone Mineral Density” or “BMD” or “Bone Density” or “Osteoporosis” or “Osteopenia”) AND (“Postmenopause” or “Post-Menopause” or “Postmenopausal”) AND (“Exercise” or “Training” or “physical exercise” or” “physical activity” or “exercise training” or “weight bearing” or “strength training” or “resistance training” or “aerobic exercise” or “isometric exercise”) AND (“Clinical trial” or “Randomized controlled trial”). Furthermore, reference lists of the included articles were searched manually to extract additional eligible articles.
The present study was conducted following the Preferred Repointing Items for Systematic Reviews and Meta-Analyses (PRISMA) approach [10]. The study was registered in the international prospective register of systematic reviews (PROSPERO ID: CRD42021241407).
Inclusion and exclusion criteria
Articles were included in this meta-analysis if they met the following inclusion criteria: (a) clinical trials with at least one exercise group as an intervention versus one control group with sedentary/habitual active lifestyle without designed exercise, (b) women with postmenopausal status at study onset, (c) intervention of at least 6 months, (d) areal BMD of the LS or/and the proximal femur regions “TH” and/or “FN” were listed as outcome measures at baseline and follow-up assessment, (e) BMD determined by dual-energy X-ray absorptiometry (DXA) or dual-photon absorptiometry (DPA), (f) ≤ 10% of participants on hormone (replacement) therapy (HT or HRT), osteoanabolic/antiresorptive (e.g., bisphosphonate, Denosumab), or osteocatabolic (glucocorticoids) and pharmaceutic agents, albeit only if the number of users was comparable between exercise and control.
The exclusion criteria were as follows: (a) mixed gender or mixed pre- and postmenopausal cohorts without separate BMD analysis for postmenopausal women; (b) women undergoing chemo- and/or radio-therapy; (c) women with diseases that relevantly affect bone metabolism; (d) interventions applying novel exercise technologies (e.g., whole-body vibration), or cycling, and swimming/aqua fitness as the only type of exercise training; (e) the synergistic/additive effect of exercise and pharmaceutic therapy; (f) double/multiple publications from one study and preliminary data from subsequently published trials; and (g) review articles, case reports, editorials, conference abstracts, letters, and unpublished reports or articles for which only abstracts were available were not considered.
Data extraction and quality assessment
Two reviewers (RM and WK) independently evaluated the full-text articles and extracted data from all the eligible publications independently. If they could not reach a consensus, a third reviewer was consulted (SvS). Information including author’s name, year of publication, country, population, number of participants, age, years since menopause, BMI, study duration, type of exercise, interventions, frequency, intensity, duration, sets and repetition, compliance, BMD values at baseline and study completion was extracted.
Two authors (RM and WK) independently assessed the risk of bias using the PEDro (Physiotherapy Evidence Database) scale [11, 12], and any discrepancy was resolved by consulting with a third reviewer (SvS). The categories assessed were randomization, allocation concealment, similarity at baseline, blinding of participants and staff, assessor blinding, incomplete outcome data, intention-to-treat analysis, between-groups comparison, and measure of variability. If a criterion was met, a point was awarded for the study; otherwise, a point was not awarded. For each trial included, a total score ranging from 0 to 10 could be obtained. The methodological quality of the included studies was classified as follows: ≥ 7 = high quality, 5–6 = moderate quality, and < 5 = low quality [13].
Outcome measures and data synthesis
The primary endpoint was the change in BMD at the LS, the femoral neck (FN), and the total hop (TH) region of interest (ROI) from baseline to follow-up. For subanalyses, the intervention was classified for (a) bone status (i.e., cohorts with versus without osteopenia/osteoporosis), (b) menopausal status of the women (i.e. early (≤ 5 years) versus late postmenopausal > 8 years (or cohort 60 years and older)) [14], and (c) supervision considering the net exercise frequency reported for the study arm. For the latter aspect, we differentiated between predominantly supervised and predominantly non-supervised exercise programs.
If the studies presented a confidence interval (CI) or standard errors (SE), these were converted to standard deviation (SD) with standardized formulas [15, 16].
Statistical analysis
We applied a random-effects meta-analysis using the metafor package [17] that is included in the statistical software R [18]. Effect size (ES) values were presented as standardized mean differences (SMDs) in combination with the 95% confidence interval (95% CI). We applied the inverse heterogeneity (IVhet) model proposed by Doi et al. [9] Heterogeneity between the studies was checked using I2 statistics. I2 of 0–40% was considered as “low,” 30–60% as “moderate,” 50–90% as “substantial,” and 75–100% as “considerable” heterogeneity [16]. Along with regression test and the rank correlation effect estimates and their standard errors using the t-test and Kendall’s τ statistic for potential publication bias, we also conducted trim and fill analyses using the L0 estimator proposed by Duval et al. [19] Additionally, we used Doi plots and the Luis Furuya-Kanamori index (LFK index) [20] to check for asymmetry. LFK values within ± 1 were considered negligible, while values ≥ ± 1 to ± 2 were considered as showing minor asymmetry. Values higher than ± 2 indicate major asymmetry. Sensitivity analyses were applied to determine whether the overall result of the analysis is robust to the use of the imputed correlation coefficient (minimum, mean or maximum). P-value < 0.05 was considered as the significance level for all the tests. SMD values of 0.2, 0.5, and 0.8 were interpreted as small, medium, and large effects.
Results
Study selection
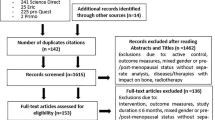
Figure 1 illustrates the search process of the study. After removing 267 duplicates, 2251 articles were screened based on title and abstract. The full texts of 101 potentially relevant articles were screened, and finally, a total of 80 articles were included in this systematic review and meta-analysis [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100]. Studies were published from 1989 to 2022 (Fig. 1). Three studies included contained English abstracts but with Italian [77, 94], Portuguese [82], and German [58] full texts.
PRISMA 2020 flow diagram for updated systematic reviews for the present project [101]
Study and participant characteristics
The 80 studies included in this systematic review and meta-analysis comprise 94 training groups and 80 control groups (Table 1). The pooled number of participants was 5581 (intervention group: 3036, control group: 2545) and sample size in individual studies ranged from 5 [77, 94] to 125 [21] participants per group. Participants in the individual studies were on average between 50 [26] and 79 [68, 102] years old. Accordingly, the average menopausal age ranges from 0.5 [91, 97] to 24 years [56]; however, many studies do not provide this important information (Table 1). The mean body mass indexes (BMI, kg/m2) of individual studies indicate that cohorts were underweight on average (e.g., [103] while others were obese [90]) (Table 1).
Difficult to rate but highly relevant for the intervention effect, 28 studies included participants with sedentary life styles, while 36 trials involved participants with some kinds of pre-study exercises activities (up to < 7 h/week [83]; Table 2). Unfortunately, many studies did not provide any information on the health and exercise status of their cohorts (Table 1).
Exercise characteristic description
Program duration varied considerably in the trials from 6 to 30 months (Table 2). Most studies (n = 42) applied an intervention period of between 9 and 18 months, while 27 trials scheduled a shorter — and 11 studies a longer — intervention period. A similarly large number of the 92 intervention groups employed either aerobic exercise (predominantly walking and/or jogging) or combined aerobic and resistance exercise as the primary exercise component. Twenty-eight interventions prescribed resistance exercise as the major component. Tai Chi was applied in five training groups [35, 69, 97, 98], hopping and jumping as the primary intervention was evaluated in six intervention groups [24, 51, 72, 77, 92]. Exercise frequency prescribed by the trials ranged from 2 sessions ([21, 34, 45, 50, 54, 70, 71, 79] to nine sessions/week [55]. The exercise session of eight studies [23, 24, 49, 77, 89, 91, 92] averaged about 10 min or less. Prescription of exercise intensity for aerobic exercise predominantly ranged between 60 and 80% of maximum heart rate (HRmax). Ground reaction forces during dynamic weight bearing exercise averaged from about ≈1.5 [48] to ≈4 × body mass [24] or potentially higher [72]. Resistance training protocols scheduled an exercise intensity of between 70 and 80% of the one repetition maximum (1-RM). In detail, four studies [26, 71, 81, 86] prescribed exercise intensities of 50% 1RM or lower. During resistance training, 1–21 exercises [42, 81, 91], with up to 108 repetitions [81], structured in 1–5 sets [22, 41, 42, 85, 91], were applied per session. Time under tension (i.e. movement velocity) was reported in only 10 studies [40, 50, 53, 59,60,61, 71, 74, 79, 87] and ranged between 3 and 9 s per repetition, with 3 studies using fast or explosive movements in the concentric part of the exercise [53, 61, 71]. In 57 exercise groups, the exercise intensity was progressively increased during the intervention period [26, 28,29,30,31, 36,37,38,39,40,41,42,43, 45,46,47, 49,50,51,52,53,54,55,56, 58,59,60,61,62,63,64,65,66,67, 71,72,73,74,75,76, 79,80,81,82, 85,86,87,88, 91, 93, 95].
Apart from one study with a very low attendance rate of 39% [84], all the other studies reported attendance rates of > 60%. Four [32, 44, 100] studies listed 100% attendance (Table 2). Unfortunately, 15 studies did not provide information on participant attendance.
Methodological quality
Methodological quality according to PEDro is shown in Table 3. Fifteen trials demonstrated high and 49 studies moderate methodological quality, while the remaining studies were classified as being of low quality (Table 3). Higher scores were frequently hindered by the lack of allocation concealment, participant, caregiver or assessor blinding, and < 85% of subjects assessed for at least one primary outcome. However, given that successful blinding of participants and caregivers (i.e., instructors) is hardly possible in exercise trials, 8 out of 10 score points can be considered an excellent result.
Outcome measures
Most of the trials determined BMD at the LS and femoral neck and/or total hip ROI. Ten studies measured BMD exclusively at the LS [46, 48, 52, 54, 55, 57, 75, 91, 95, 100], and seven studies determined BMD only at the proximal femur [25, 49, 62, 66, 74, 89, 94].
Meta-analysis results
Effects of exercise on BMD at the lumbar spine
Eighty-five comparisons addressed exercise effects at BMD-LS (Fig. 2). In summary, the inverse heterogeneity model (IVhet) (Fig. 2) with imputation of the mean correlation demonstrated a significant effect (p < 0.001) of exercise on BMD at the LS (SMD: 0.29; 95% CI: 0.16 to 0.42). Heterogeneity between the trial results (I2 = 68%) can be classified as substantial (Fig. 2). Applying sensitivity analysis with imputation with minimum correlation (i.e., maximum SD, SMD: 0.21; 95% CI: 0.12 to 0.29) or maximum correlation (i.e., minimum SD, SMD: 0.45; 95% CI: 0.21 to 0.69) led to diverging, but consistently significant (p < 0.001) results.
The IVhet model–based funnel plot analysis with trim and fill suggests significant evidence for a publication/small study bias (Fig. 3). The analysis imputes nine missing studies on the left-hand side (i.e., favors control group). However, even the corrected, i.e., imputation-adjusted, intervention effect remains significant (p = 0.008). The significant asymmetry was confirmed by the LFK Index (1.50), the regression (p = 0.011), and the rank correlation test (p = 0.016).
Effects of exercise on BMD at the femoral neck
Thirty-one group comparisons determined exercise effects at BMD-FN (Fig. 4). In summary, the IVhet model with imputation of the mean correlation demonstrated a significant effect (p < 0.001) of exercise on BMD at the FN (SMD: 0.27; 95% CI: 0.16 to 0.39) (Fig. 4). Heterogeneity between the trial results (I2 = 58%) was moderate (Fig. 4). Applying imputation with minimum correlation (SMD: 0.20; 95% CI: 0.13 to 0.27) or maximum correlation (SMD: 0.55; 95% CI: 0.24 to 0.86) led to diverging, but consistently significant (p < 0.001), results.
The funnel plot analysis with trim and fill suggests no relevant evidence for a publication/small study bias (Fig. 5). Inspecting the LFK Index (1.04), we observed minor asymmetry; results of the regression (p = 0.068) and rank correlation test (p = 0.127) were non-significant, however.
Effects of exercise on BMD at the total hip
Thirty comparisons addressed exercise effects for TH BMD (Fig. 6). The IVhet model (Fig. 6) with imputation of the mean correlation revealed a significant effect (p < 0.001) of exercise on BMD at the TH (SMD: 0.41; 95% CI: 0.30 to 0.52). Heterogeneity between the trial results (I2 = 20%) was low (Fig. 6). Applying sensitivity analyses with imputation with minimum correlation (SMD: 0.31; 95% CI: 0.22 to 0.42) or maximum correlation (SMD: 0.64; 95% CI: 0.44 to 0.83) led to diverging, but consistently significant (p < 0.001), results.
The funnel plot for TH BMD suggested evidence for a small study/publication bias (Fig. 7). The trim and fill model imputed three studies at the lower right-hand side (i.e., small-moderate sized studies with positive results). Considering these (imputed) data in the analysis, SMD increased slightly (0.43; 95% CI: 0.32–0.54). Funnel plot asymmetry was not confirmed by the LFK index (− 0.04), regression (p = 0.47), or rank test (p = 0.57).
Subanalyses on potentially modifying factors
Effect of bone status
Fourteen of 85 comparisons focused on BMD-LS in cohorts with osteopenia, osteoporosis, or a history of fractures (Table 1). The IVhet model determined no significant difference (p = 0.094) between the subgroups with (SMD: 0.54; 95% CI: 0.17 to 0.92) and without (SMD: 0.23; 95% CI: 0.08 to 0.38) osteopenia/osteoporosis on LS-BMD. Heterogeneity was substantial (I2: 63% and I2: 77%) in both subgroups. Ten versus 62 subgroups with and without osteopenia/osteoporosis were compared for FN. In summary, we observed slightly more favorable effects in the cohorts with osteopenia, osteoporosis, or a history of fractures; the between group differences were not significant (p = 0.711), however. The same was true for TH-BMD (p = 0.453) with 6 versus 25 comparisons.
Effect of menopausal status
In summary, we compared 7 early postmenopausal with 26 late postmenopausal study groups for LS-, 5 with 26 subgroups for FN-, and 5 with 9 subgroups for TH-BMD. In summary, we detected no significant BMD-difference at the LS (p = 0.901), FN (p = 0.547), or TH-BMD (p = 0.824).
Effect of supervision on exercise effects on BMD in postmenopausal women
Fifty-nine study groups that addressed LS-BMD applied a predominantly supervised exercise protocol, while 23 study arms focused on predominantly non-supervised exercise. In summary, the supervised exercise protocols revealed only tendentially (p = 0.37) higher effects compared with the predominantly unsupervised exercise protocols on LS-BMD (SMD: 0.19; 95% CI: − 0.03 to 0.41). In parallel, we observed no significant differences (p = 0.549) between predominantly supervised (n = 50) versus non-supervised (n = 19) exercise protocols for FN-BMD. However, the predominantly non-supervised exercise study groups per se did not show a significant exercise effect on BMD-LS and FN (p = 0.09 each). Finally, we observed no significant differences in TH-BMD (p = 0.798) for predominantly supervised (n = 25) versus predominantly non-supervised protocols (n = 5) with significant exercise effects for both subgroups.
Discussion
In the present systematic review and meta-analysis, we provide further evidence for the favourable effect of exercise on BMD at LS, FN, and TH in postmenopausal women. However, since this 2022 update added six exercise trials [51, 53, 54, 72, 77, 96] while excluding one (aquatic exercise) trial [104], the result of significant but “small” exercise effects at LS (SMD: 0.29), FN (SMD: 0.27), and TH (SMD: 0.41) did not differ relevantly from our 2020 finding [1]. However, one main advantage of the present study is the application of the inverse heterogeneity model (IVhet) [9]. The IVhet approach is less susceptible to underestimation of statistical error in heterogeneous studies, i.e., results are more reliable in heterogeneous studies especially with respect to the coverage probability of confidence intervals [105]. Nevertheless, it would be a misconception to assume that the enormous heterogeneity between the trial results can be adequately addressed by statistical methods. Of course, other and our (e.g., [106,107,108,109]) meta-analytic results are simply the quintessence taken from a pool of studies with favorable and less favorable results. This is generally the case for meta-analyses; however, the situation in the field of exercise and (particularly) bone strength is much more complex than the sophisticated pharmacologic area, where this type of analysis was first applied. It is obvious that in contrast to pharmaceutic studies, the vast majority of exercise studies started immediately with the phase III study approach, without having addressed the general effectiveness of the exercise protocol in earlier pilot studies [110]. In some cases, one gains the impression that less promising interventions were applied to verify their ineffectiveness to favorably affect bone. Since there is no reliable rationale to exclude these “just look what happens trials,” the dilution of the meta-analytic results is predictable.
Another enormous problem of systematic reviews and meta-analyses in the area of exercise and bone strength is the extreme variation among the participants and intervention characteristics of the individual trials. Considering the first aspect, bone [4, 111] and menopausal status [58] might be candidates with potentially moderating effect of exercise effect on BMD. In summary, we did not, however, observe any significant differences on BMD at LS, FN, and TH between the subgroups, be it for bone or for menopausal status. Of importance, with few exceptions (e.g., menopausal status: subgroup-analyses for TH-ROI), the number of studies included in the subgroups was high enough to exclude a predominate random effect. Nevertheless, there is some evidence that interaction effects between participant and exercise parameters in particular have affected our findings. As an example, it is plausible that more intense exercise protocols were applied in the younger, i.e., early postmenopausal cohorts or participants with a lower fracture risk, i.e., participants without osteopenia/osteoporosis. Reviewing the studies, however, we observed no striking differences in exercise intensity (or training frequency) between the cohorts with diverging bone or menopausal status. Even so, a simple adjustment of the subgroup analysis for exercise intensity is debatable, since the relevance of exercise intensity on a given outcome depends on other variables such as training frequency. The most successful approach for addressing the impact of participant characteristics such as bone or menopausal status on exercise induced BMD-changes might thus be to include participants with diverging characteristics but to apply the identical protocol.
While the effect of bone and menopausal status on exercise effect in middle aged-older women are “nice to know” for the exercise specialist, supervision of the exercise protocol is an issue with significant implications for various crucial elements of an intervention including setting, personal, finances, facilities, participants, and exercise protocol. This led us to the present approach of determining the effect of supervision on the exercise program on BMD. In a recent publication, we observed a significant superiority of supervision (versus predominantly non-supervised protocols) on overall and main osteoporotic fracture incidence in middle-aged to older adults [7]. This result was supported by findings [5, 112] which reported that supervised protocols demonstrate significantly higher effects on dynamic balance, strength, and power, i.e., parameters related to fall risk [113, 114] and bone strength [8]. Fisher et al. [5] postulated that the superiority of supervised (resistance) exercise programs might be related to higher adherence, motivation, intensity progression, and safety. Exercise programs on bone strengthening applied intensive resistance, weight bearing, and impact exercises [115, 116] which also underline the relevance of supervision for effective and safe exercise protocols, especially for older people. However, our approach does not determine a significant difference between predominantly supervised versus non-supervised exercise protocols, albeit with slightly higher effects of supervised protocols for BMD at the LS, FN, and TH. Again, we have to admit that in particular exercise parameters related to supervision (or not) diluted our result, e.g., some types of exercise (i.e. dynamic resistance exercise) need higher degrees of supervision compared to others (i.e. jumping protocols). Nevertheless, accepting this result as a reliable finding would simplify the broad implementation of exercise programs in the field of osteoporosis due to lower demands on personnel and — since most non-supervised protocols applied home exercise — locations. However, the decision about more or less supervision should ultimately be made in the light of the cohort addressed (e.g., fitness and health status), specific preventative/therapeutic aims and budget considerations in order to generate the safest and most effective/efficient training setting.
Apart from supervision, one may argue that comprehensive meta-analyses or their subanalyses on crucial exercise characteristics (i.e., type of exercise, strain intensity, exercise frequency, etc.) [1, 117] might be a smart solution for generating reliable recommendations on exercise protocols. In the meantime and in line with Gentil et al. [118], we do no longer agree with this idea. In fact, the close and inherent interactions within the given exercise protocol aggravate the addressing of isolated exercise parameters. For example, exercise intensity plays an important role for bone strengthening [117], but its relevance is (among other things) dependent on training frequency (once per day or once per week?) [119]. Bearing in mind that the effectiveness of continuously unchanged stimuli is limited [120], the aspect of progression is also crucial in particular when applying year-long exercise interventions [121]. One solution to this problem might be comparative meta-analyses that include trials with two study arms with diverging exercise parameters (e.g. intensity [122] or training frequency [119]) but otherwise identical protocols (… and participants).
At this point, we would like to briefly address some limitations and particularities of the present work. (1) Apart from the general limitation that quite heterogeneous exercise protocols have to be included in the analysis, some of our eligibility criteria might be also debatable. This refers to study length (≥ 6 months) and adjuvant pharmaceutic therapy with impact on bone metabolism (≤ 10% of participants — albeit only if the number of users was similar between exercise and control). Although evidence for additive effects of exercise and hormone replacement therapy [123] or bisphosphonates [124] is low, we tried to exclude such interactions. Furthermore, considering bone remodeling as the primary mode of bone renewal in adults [125, 126] and taking regular changes of exercise intensity into account, exercise studies shorter than 6 months might not have reached the full amount of mineralized bone and thus confound the BMD assessment. (2) In this context, a limitation to be mentioned was that only few data were available on the precision of DXA measurements and the calculated least significant change (LSC). The minimum acceptable precision for an individual technologist would be 1.9% (LSC = 5.3%) for BMD-LS, 1.8% (LSC = 5.0%) for BMD-TH and 2.5% (LSC = 6.9%) for BMD-FN [127]. (3) Another predominantly biometrical source of error was that SDs of the absolute change in BMD were not consistently available and thus had to be imputed. Although sensitivity analysis on imputation strategy consistently showed significant effects, outcomes for BMD at the LS, FN and TH varied considerably depending on whether imputation was conducted with mean, minimum, or maximum correlation. On the other hand, inspecting funnel and Doi plots, LFK indices, rank, and regression tests led to the conclusion that the probability of small study effects (publication bias, outcome reporting bias, dissemination bias, etc.) with confounding effects on our finding [128] is limited. (4) Our subgroup analyses on exercise-induced BMD changes focus on bone and menopausal status and supervision of the exercise session. We think that these are important aspects to be addressed, but other participant or study characteristics, i.e., baseline physical activity/exercise status or site specifity of the exercise might be equally or even more important and should be addressed by future studies. (5) Our subgroup analysis on menopausal status allocated study groups ≤ 5 years postmenopausal to the early and study groups > 8 years postmenopausal (or cohorts 60 years and older) to the late postmenopausal subgroup. Considering the considerable individual variation around the average time of menopause of 51 years [129], including study groups with a lower range of 60 years of age might slightly confound our analysis since few subjects of these cohorts might not fulfill the criteria of 8 years postmenopause. Nevertheless, an analysis with an alternative classification (i.e., ≤ 8 years postmenopausal: early versus > 8 years: late postmenopausal) results in similar non-significant group differences. (6) Although we carefully examined the studies, classification on “predominantly supervised” versus “predominantly non-supervised” based on attendance rates might be difficult and not always meaningful. This becomes more apparent where, for example, a study protocol that applied up to two supervised DRT and WB exercise sessions and three non-supervised walking sessions/week was classified as “predominantly non-supervised” by our procedure (5).
By applying robust statistical methods (i.e., the IVhet approach [9] which is less susceptible to underestimation of statistical error in the heterogeneous exercise studies), we provided further evidence for a positive effect of exercise on BMD at LS, FN, and TH in postmenopausal women. However, the average SMD for BMD effects on LS, FN, and TH can be classified as moderate at best (i.e., 0.2 to 0.5). Our subanalysis on bone and menopausal status and supervision effects did not indicate significant differences. Summing up our recent experiences and findings, we conclude that while comprehensive meta-analyses in the area of exercise and bone strength might be useful for a rapid (but rough) overview, their practical application for deriving dedicated and reliable exercise recommendations is rather limited.
Data availability
The data that support the findings of this study are available from the corresponding author (WK), upon reasonable request.
References
Shojaa N, von Stengel S, Schoene D et al (2020) Effect of exercise training on bone mineral density in postmenopausal women: a systematic review and meta-analysis of intervention studies. Front Physiol 11:1427–1444. https://doi.org/10.3389/fphys.2020.00652
Nohara T, Kamei T, Ohta A (2006) Accelerated decrease in bone mineral density in women aged 52–57 years. Tohoku J Exp Med 210:341–347
Shipman AJ, Guy GW, Smith I, Ostlere S, Greer W, Smith R (1999) Vertebral bone mineral density, content and area in 8789 normal women aged 33–73 years who have never had hormone replacement therapy. Osteoporos Int 9:420–426
Kemmler W, Riedel H (1998) Einfluß eines intensiven 9monatigen körperlichen Trainings auf Knochendichte, Gesamtkalzium und Wirbelkörperbreite bei Frauen mit Osteoporose, Osteopenie und Knochengesunden. Osteologie 7:203–210
Fisher J, Steele J, Wolf M, Androulakis-Korakakis P, Smith D, Giessing J, Wescott WL (2021) The role of supervision in resistance training; an exploratory systematic review and meta-analysis. Sportrxiv. https://doi.org/10.51224/SRXIV.18
Franco M, Howard K, Sherrington C et al (2015) Eliciting older people’s preferences for exercise programs: a best-worst scaling choice. J Physiother 61:34–41
Hoffmann I, Shojaa M, Kohl M et al (2022) Exercise reduces the number of overall and major osteoporotic fractures in adults. Does supervision make a difference? Systematic review and meta-analysis. J Bone Miner Res 37(11):2132–2148. https://doi.org/10.1002/jbmr.4683
Kemmler W, Stengel V (eds) (2019) The role of exercise on fracture reduction and bone strengthening. Avademic Press, London
Doi SA, Barendregt JJ, Khan S, Thalib L, Williams GM (2015) Advances in the meta-analysis of heterogeneous clinical trials I: the inverse variance heterogeneity model. Contemp Clin Trials 45:130–138. https://doi.org/10.1016/j.cct.2015.05.009
Moher D, Shamseer L, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4:1. https://doi.org/10.1186/2046-4053-4-1
de Morton NA (2009) The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother 55:129–133. https://doi.org/10.1016/S0004-9514(09)70043-1
Sherrington C, Herbert RD, Maher CG, Moseley AM (2000) PEDro. A database of randomized trials and systematic reviews in physiotherapy. Man Ther 5:223–226. https://doi.org/10.1054/math.2000.0372
Ribeiro de Avila V, Bento T, Gomes W, Leitao J, Fortuna de Sousa N (2018) Functional outcomes and quality of life after ankle fracture surgically treated: a systematic review. J Sport Rehabil 27:274–283. https://doi.org/10.1123/jsr.2016-0199
Harlow SD, Gass M, Hall JE et al (2012) Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause 19:387–395. https://doi.org/10.1097/gme.0b013e31824d8f40
Higgins J, Thomas J, Chandler J et al (2021) Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane 2021
Higgins JP, Altman DG, Gotzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor Package. J Stat Softw 36:1–48. https://doi.org/10.18637/jss.v036.i03
R_Development_Core_Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Duval SJ, Tweedie RL (2000) A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. JASA 95:89–98. https://doi.org/10.1080/01621459.2000.10473905
Furuya-Kanamori L, Barendregt JJ, Doi SAR (2018) A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc 16:195–203. https://doi.org/10.1097/XEB.0000000000000141
Adami S, Gatti D, Braga V, Bianchini D, Rossini M (1999) Site-specific effects of strength training on bone structure and geometry of ultradistal radius in postmenopausal women. J Bone Miner Res 14:120–124
Basat H, Esmaeilzadeh S, Eskiyurt N (2013) The effects of strengthening and high-impact exercises on bone metabolism and quality of life in postmenopausal women: a randomized controlled trial. J Back Musculoskelet Rehabil 26:427–435. https://doi.org/10.3233/BMR-130402
Bassey EJ, Ramsdale SJ (1995) Weight-bearing exercise and ground reaction forces: a 12-month randomized controlled trial of effects on bone mineral density in healthy postmenopausal women. Bone 16:469–476. https://doi.org/10.1016/8756-3282(95)90193-0
Bassey EJ, Rothwell MC, Littlewood JJ, Pye DW (1998) Pre- and postmenopausal women have different bone mineral density responses to the same high-impact exercise. J Bone Miner Res 13:1805–1813
Bello M, Sousa MC, Neto G et al (2014) The effect of a long-term, community-based exercise program on bone mineral density in postmenopausal women with pre-diabetes and type 2 diabetes. J Hum Kinet 43:43–48. https://doi.org/10.2478/hukin-2014-0088
Bemben DA, Fetters NL, Bemben MG, Nabavi N, Koh ET (2000) Musculoskeletal responses to high- and low-intensity resistance training in early postmenopausal women. Med Sci Sports Exerc 32:1949–1957
Bemben DA, Palmer IJ, Bemben MG, Knehans AW (2010) Effects of combined whole-body vibration and resistance training on muscular strength and bone metabolism in postmenopausal women. Bone 47:650–656. https://doi.org/10.1016/j.bone.2010.06.019
Bergström I, Landgren BM, Brinck J, Freyschuss B (2008) Physical training preserves bone mineral density in postmenopausal women with forearm fractures and low bone mineral density. Osteoporos Int 19:177–183
Bloomfield SA, Williams NI, Lamb DR, Jackson RD (1993) Non-weightbearing exercise may increase lumbar spine bone mineral density in healthy postmenopausal women. Am J Phys Med Rehabil 72:204–209
Bocalini DS, Serra AJ, dos Santos L, Murad N, Levy RF (2009) Strength training preserves the bone mineral density of postmenopausal women without hormone replacement therapy. J Aging Health 21:519–527. https://doi.org/10.1177/0898264309332839
Bolton KL, Egerton T, Wark J et al (2012) Effects of exercise on bone density and falls risk factors in post-menopausal women with osteopenia: a randomised controlled trial. J Sci Med Sport 15:102–109. https://doi.org/10.1016/j.jsams.2011.08.007
Brooke-Wavell K, Jones PR, Hardman AE (1997) Brisk walking reduces calcaneal bone loss in post-menopausal women. Clin Sci (Lond) 92:75–80
Brooke-Wavell K, Jones PR, Hardman AE, Tsuritan YY (2001) Commencing, continuing and stopping brisk walking: effects on bone mineral density, quantitative ultrasound of bone and markers of bone metabolism in postmenopausal women. Osteoporos Int 12:581–587
Caplan GA, Ward JA, Lord SR (1993) The benefits of exercise in postmenopausal women. Aust J Public Health 17:23–26
Chan K, Qin L, Lau M et al (2004) A randomized, prospective study of the effects of Tai Chi Chun exercise on bone mineral density in postmenopausal women. Arch Phys Med Rehabil 85:717–722
Chilibeck PD, Davison KS, Whiting SJ, Suzuki Y, Janzen CL, Peloso P (2002) The effect of strength training combined with bisphosphonate (etidronate) therapy on bone mineral, lean tissue, and fat mass in postmenopausal women. Can J Physiol Pharmacol 80:941–950
Chilibeck PD, Vatanparast H, Pierson R et al (2013) Effect of exercise training combined with isoflavone supplementation on bone and lipids in postmenopausal women: a randomized clinical trial. J Bone Miner Res 28:780–793. https://doi.org/10.1002/jbmr.1815
Choquette S, Riesco E, Cormier E, Dion T, Aubertin-Leheudre M, Dionne IJ (2011) Effects of soya isoflavones and exercise on body composition and clinical risk factors of cardiovascular diseases in overweight postmenopausal women: a 6-month double-blind controlled trial. Br J Nutr 105:1199–1209. https://doi.org/10.1017/s0007114510004897
Chuin A, Labonte M, Tessier D et al (2009) Effect of antioxidants combined to resistance training on BMD in elderly women: a pilot study. Osteoporos Int 20:1253–1258. https://doi.org/10.1007/s00198-008-0798-5
de Matos O, Lopes da Silva DJ, Martinez de Oliveira J, Castelo-Branco C (2009) Effect of specific exercise training on bone mineral density in women with postmenopausal osteopenia or osteoporosis. Gynecol Endocrinol 25: 616–620. 912462217 [pii] https://doi.org/10.1080/09513590903015593
Deng S (2009) Effects of exercise therapy on bone mineral density in early postmenopausal women: a controlled trial. Front Med China 3:323–329
de Oliveira LC, de Oliveira RG, de Almeida Pires-Oliveira DA (2019) Effects of whole-body vibration versus pilates exercise on bone mineral density in postmenopausal women: a randomized and controlled clinical trial. J Geriatr Phys Ther 42:E23–E31. https://doi.org/10.1519/JPT.0000000000000184
Duff WR, Kontulainen SA, Candow DG et al (2016) Effects of low-dose ibuprofen supplementation and resistance training on bone and muscle in postmenopausal women: A randomized controlled trial. Bone Rep 5:96–103. https://doi.org/10.1016/j.bonr.2016.04.004
Ebrahim SB, Thompson PW, Baskaran VKE (1997) Randomized placebo controlled trial of brisk walking in the prevention of postmenopausal osteoporosis. Age Aging 26:252–260
Englund U, Littbrand H, Sondell A, Pettersson U, Bucht G (2005) A 1-year combined weight-bearing training program is beneficial for bone mineral density and neuromuscular function in older women. Osteoporos Int 16:1117–1123
Evans EM, Racette SB, Van Pelt RE, Peterson LR, Villareal DT (2007) Effects of soy protein isolate and moderate exercise on bone turnover and bone mineral density in postmenopausal women. Menopause 14:481–488
Going S, Lohman T, Houtkooper L et al (2003) Effects of exercise on bone mineral density in calcium-replete postmenopausal women with and without hormone replacement therapy. Osteoporos Int 14:637–643
Grove KA, Londeree BR (1992) Bone density in postmenopausal women: high impact vs low impact exercise. Med Sci Sports Exerc 24:1190–1194
Hans D, Genton L, Drezner MK et al (2002) Monitored impact loading of the hip: initial testing of a home-use device. Calcif Tissue Int 71:112–120
Hartard M, Haber P, Ilieva D, Preisinger E, Huber JC (1996) Systematic strength training as a model of therapeutic intervention. Arch Phys Med Rehabil 75:21–28
Hartley C, Folland JP, Kerslake R, Brooke-Wavell K (2020) High-impact exercise increased femoral neck bone density with no adverse effects on imaging markers of knee osteoarthritis in postmenopausal women. J Bone Miner Res 35:53–63. https://doi.org/10.1002/jbmr.3867
Hatori M, Hasegawa A, Adachi H et al (1993) The effects of walking at the anaerobic threshold level on vertebral bone loss in postmenopausal women. Calcif Tissue Int 52:411–414
Hettchen M, von Stengel S, Kohl M et al (2021) Changes in menopausal risk factors in early postmenopausal osteopenic women after 13 months of high-intensity exercise: the randomized controlled ACTLIFE-RCT. Clin Interv Aging 16:83–96. https://doi.org/10.2147/CIA.S283177
Holubiac IS, Leuciuc FV, Craciun DM, Dobrescu T (2022) Effect of strength training protocol on bone mineral density for postmenopausal women with osteopenia/osteoporosis assessed by dual-energy x-ray absorptiometry (DEXA). Sens (Basel) 22(5):1904. https://doi.org/10.3390/s22051904
Iwamoto J, Takeda T, Ichimura S (2001) Effects of exercise training and detraining on bone mineral density in postmenopausal women with osteoporosis. J Orthop Sci 6:128–132
Jessup JV, Horne C, Vishen RK, Wheeler D (2003) Effects of exercise on bone density, balance, and self-efficacy in older women. Biol Res Nurs 4:171–180. https://doi.org/10.1177/1099800402239628
Karakiriou S, Douda H, Smilios I, Volaklis KA, Tokmakidis SP (2012) Effects of vibration and exercise training on bone mineral density and muscle strength in post-menopausal women. Eur JSport Sci 12:81–88. https://doi.org/10.1080/17461391.2010.536581
Kemmler W (1999) Einfluß unterschiedlicher Lebensabschnitte auf die belastungsabhängige Reaktion ossärer Risikofaktoren einer Osteoporose. Dtsch Z Sportmed 50:114–119
Kemmler W, Lauber D, Weineck J, Hensen J, Kalender W, Engelke K (2004) Benefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women: results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS). Arch Intern Med 164:1084–1091. https://doi.org/10.1001/archinte.164.10.1084/164/10/1084
Kemmler W, von Stengel S, Engelke K, Haberle L, Kalender WA (2010) Exercise effects on bone mineral density, falls, coronary risk factors, and health care costs in older women: the randomized controlled senior fitness and prevention (SEFIP) study. Arch Intern Med 170: 179–185. 170/2/179 [pii] https://doi.org/10.1001/archinternmed.2009.499
Kemmler W, Bebenek M, von Stengel S, Engelke K, Kalender WA (2013) Effect of block-periodized exercise training on bone and coronary heart disease risk factors in early post-menopausal women: a randomized controlled study. Scand J Med Sci Sports 23:121–129. https://doi.org/10.1111/j.1600-0838.2011.01335.x
Kerr D, Morton A, Dick I, Prince R (1996) Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent. J Bone Miner Res 11:218–225
Kerr D, Ackland T, Maslen B, Morton A, Prince R (2001) Resistance training over 2 years increases bone mass in calcium-replete postmenopausal women. J Bone Miner Res 16:175–181
Kohrt WM, Snead DB, Slatopolsky E, Birge SJ Jr (1995) Additive effects of weight-bearing exercise and estrogen on bone mineral density in older women. J Bone Miner Res 10:1303–1311
Kohrt WM, Ehsani AA, Birge SJ (1997) Effects of exercise involving predominantly either joint-reaction or ground-reaction forces on bone mineral density in older women. J Bone Miner Res 12:1253–1261
Korpelainen R, Keinanen-Kiukaanniemi S, Heikkinen J, Vaananen K, Korpelainen J (2006) Effects of impact exercise on bone mineral density in elderly women with low BMD: a population based randomized controlled 30-month intervention. Osteoporos Int 17:109–118
Kwon Y, Park SK, Kim E, Park J (2008) The effects of multi-component exercise training on VO2max, muscle mass, whole bone mineral density and fall risk in community-dwelling elderly women. Jpn J Phys Fit Sport 57:339–348
Lau EM, Woo J, Leung PC, Swaminathan R, Leung D (1992) The effects of calcium supplementation and exercise on bone density in elderly Chinese women. Osteoporos Int 2:168–173
Liu BX, Chen SP, Li YD et al (2015) The effect of the modified eighth section of eight-section brocade on osteoporosis in postmenopausal women: a prospective randomized trial. Medicine (Baltimore) 94:e991. https://doi.org/10.1097/MD.0000000000000991
Lord SR, Ward JA, Williams P, Zivanovic E (1996) The effect of a community exercise program on fracture risk factors in older women. Osteoporos Int 6:361–367
Maddalozzo GF, Widrick JJ, Cardinal BJ, Winters-Stone KM, Hoffman MA, Snow CM (2007) The effects of hormone replacement therapy and resistance training on spine bone mineral density in early postmenopausal women. Bone 40:1244–1251
Marin-Cascales E, Rubio-Arias JA, Alcaraz PE (2019) Effects of two different neuromuscular training protocols on regional bone mass in postmenopausal women: a randomized controlled trial. Front Physiol 10:846. https://doi.org/10.3389/fphys.2019.00846
Marques EA, Mota J, Machado L et al (2011) Multicomponent training program with weight-bearing exercises elicits favorable bone density, muscle strength, and balance adaptations in older women. Calcif Tissue Int 88:117–129. https://doi.org/10.1007/s00223-010-9437-1
Marques EA, Wanderley F, Machado L et al (2011) Effects of resistance and aerobic exercise on physical function, bone mineral density, OPG and RANKL in older women. Exp Gerontol 46: 524–532. S0531-5565(11)00055-6 [pii] https://doi.org/10.1016/j.exger.2011.02.005
Martin D, Notelovitz M (1993) Effects of aerobic training on bone mineral density of postmenopausal women. J Bone Miner Res 8:931–936
Milliken LA, Going SB, Houtkooper LB et al (2003) Effects of exercise training on bone remodeling, insulin-like growth factors, and bone mineral density in postmenopausal women with and without hormone replacement therapy. Calcif Tissue Int 72:478–484
Montgomery GJ, Abt G, Dobson CA, Evans WJ, Aye M, Ditroilo M (2020) A 12-month continuous and intermittent high-impact exercise intervention and its effects on bone mineral density in early postmenopausal women: a feasibility randomized controlled trial. J Sports Med Phys Fitness 60:770–778. https://doi.org/10.23736/S0022-4707.20.10412-2
Nelson ME, Fisher EC, Dilmanian FA, Dallal GE, Evans WJ (1991) A 1-y walking program and increased dietary calcium in postmenopausal women: effects on bone. Am J Clin Nutr 53:1304–1311
Nelson ME, Fiatarone MA, Morganti CM, Trice I, Greenberg RA, Evans WJ (1994) Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. A andomized controlled trial. Jama 272:1909–1914
Nichols JF, Nelson KP, Peterson KK, Satoris DJ (1995) Bone Mineral density responses to high-intensity strength training in older women. J Age Phys Act 3:26–38
Nicholson VP, McKean MR, Slater GJ, Kerr A, Burkett BJ (2015) Low-load very high-repetition resistance training attenuates bone loss at the lumbar spine in active post-menopausal women. Calcif Tissue Int 96:490–499. https://doi.org/10.1007/s00223-015-9976-6
Orsatti F, Petri-Nahas E, Nahas-Neto J, Orsatti C, Teixeira A (2013) Effects of isoflavone and counter-resistance training on bone mineral density in postmenopausal women. Rev Bras Cineantropom Desempenho Hum 15:726–736
Park H, Kim KJ, Komatsu T, Park SK, Mutoh Y (2008) Effect of combined exercise training on bone, body balance, and gait ability: a randomized controlled study in community-dwelling elderly women. J Bone Miner Metab 26:254–259. https://doi.org/10.1007/s00774-007-0819-z
Prince RL, Devine A, Dick I et al (1995) The effect of calcium supplementation (milk powder or tablets) and exercise on bone density in postmenopausal women. J Bone Miner Res 10:1068–1075
Pruitt LA, Jackson RD, Bartels RL, Lehnhard HJ (1992) Weight-training effects on bone mineral density in early postmenopausal women. J Bone Miner Res 7:179–185
Pruitt LA, Taaffe DR, Marcus R (1995) Effects of a one-year high-intensity versus low-intensity resistance training program on bone mineral density in older women. J Bone Miner Res 10:1788–1795
Rhodes EC, Martin AD, Taunton JE, Donnelly M, Warren J, Elliot J (2000) Effects of one year of resistance training on the relation between muscular strength and bone density in elderly women. Br J Sports Med 34:18–22
Ryan AS, Nicklas BJ, Dennis KE (1998) Aerobic exercise maintains regional bone mineral density during weight loss in postmenopausal women. J Appl Physiol 84:1305–1310
Sakai A, Oshige T, Zenke Y, Yamanaka Y, Nagaishi H, Nakamura T (2010) Unipedal standing exercise and hip bone mineral density in postmenopausal women: a randomized controlled trial. J Bone Miner Metab 28:42–48. https://doi.org/10.1007/s00774-009-0100-8
Silverman NE, Nicklas BJ, Ryan AS (2009) Addition of aerobic exercise to a weight loss program increases BMD, with an associated reduction in inflammation in overweight postmenopausal women. Calcif Tissue Int 84:257–265. https://doi.org/10.1007/s00223-009-9232-z
Sinaki M, Wahner HW, Offord KP, Hodgson SF (1989) Efficacy of nonloading exercises in prevention of vertebral bone loss in postmenopausal women: a controlled trial. Mayo Clin Proc 64:762–769
Sugiyama T, Yamaguchi A, Kawai S (2002) Effects of skeletal loading on bone mass and compensation mechanism in bone: a new insight into the “mechanostat” theory. J Bone Miner Metab 20:196–200
Tartibian B, Hajizadeh Maleki B, Kanaley J, Sadeghi K (2011) Long-term aerobic exercise and omega-3 supplementation modulate osteoporosis through inflammatory mechanisms in post-menopausal women: a randomized, repeated measures study. Nutr Metab (Lond) 8:71. https://doi.org/10.1186/1743-7075-8-71
Tolomio S, Lalli A, Travain G, Zaccaria M (2009) Effects of a combined weight- and non weight-bearing (water) exercise program on bone mass and quality in postmenopausal women with low bone mineral density. Clin Ter 160:105–109
Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S (2004) Effects of 6-month whole body vibration training on hip density; muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Min Res 19:352–359
Waltman N, Kupzyk KA, Flores LE, Mack LR, Lappe JM, Bilek LD (2022) Bone-loading exercises versus risedronate for the prevention of osteoporosis in postmenopausal women with low bone mass: a randomized controlled trial. Osteoporos Int 33:475–486. https://doi.org/10.1007/s00198-021-06083-2
Wang H, Yu B, Chen W, Lu Y, Yu D (2015) Simplified Tai Chi resistance training versus traditional Tai Chi in slowing bone loss in postmenopausal women. Evid Based Complement Alternat Med 2015:379451. https://doi.org/10.1155/2015/379451
Woo J, Hong A, Lau E, Lynn H (2007) A randomised controlled trial of Tai Chi and resistance exercise on bone health, muscle strength and balance in community-living elderly people. Age Ageing 36: 262–268. afm005 [pii] https://doi.org/10.1093/ageing/afm005
Wu J, Oka J, Tabata I et al (2006) Effects of isoflavone and exercise on BMD and fat mass in postmenopausal Japanese women: a 1-year randomized placebo-controlled trial. J Bone Miner Res 21:780–789
Yamazaki S, Ichimura S, Iwamoto J, Takeda T, Toyama Y (2004) Effect of walking exercise on bone metabolism in postmenopausal women with osteopenia/osteoporosis. J Bone Miner Metab 22:500–508. https://doi.org/10.1007/s00774-004-0514-2
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Tella SH, Gallagher JC (2014) Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol 142:155–170. https://doi.org/10.1016/j.jsbmb.2013.09.008
Iwamoto J, Takeda T, Ichimura S (1998) Effect of exercise on tibial and lumbar vertebral bone mass in mature osteopenic rats: bone histomorphometry study. J Orthop Sci 3:257–263
Moreira LD, Fronza FC, Dos Santos RN et al (2014) The benefits of a high-intensity aquatic exercise program (HydrOS) for bone metabolism and bone mass of postmenopausal women. J Bone Miner Metab 32:411–419. https://doi.org/10.1007/s00774-013-0509-y
Furuya-Kanamori L, Thalib L, Barendregt J (2017) Meta-analysis in evidence-based healthcare: a paradigm shift away from random effects is overdue. Int J Evid 15:152–160. https://doi.org/10.1097/XEB.0000000000000125
Howe TE, Shea B, Dawson LJ et al (2011) Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev CD000333. https://doi.org/10.1002/14651858.CD000333.pub2
Marques EA, Mota J, Carvalho J (2011) Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age 34:1493–1515. https://doi.org/10.1007/s11357-011-9311-8
Martyn-St James M, Carroll S (2009) A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med 43: 898–908. bjsm.2008.052704 [pii] https://doi.org/10.1136/bjsm.2008.052704
Martyn-St James M, Caroll S (2006) High intensity resistance training and postmenopausal bone loss: a meta-analysis. Osteoporos Int 17:1225–1240
Kemmler W, Fröhlich M, Pieter A, Mayerl J (2020) Evidenz und Evidenzbasierte Praxis. In: Fröhlich M, Mayerl J, Pieter A, Kemmler W (eds) Einführung in die Methoden, Methodologie und Statistik im Sport. Springer Nature, Berlin, pp 109–113
Souza D, Barbalho M, Ramirez-Campillo R, Martins W, Gentil P (2020) High and low-load resistance training produce similar effects on bone mineral density of middle-aged and older people: a systematic review with meta-analysis of randomized clinical trials. Exp Gerontol 138:110973. https://doi.org/10.1016/j.exger.2020.110973
Lacroix A, Hortobagyi T, Beurskens R, Granacher U (2017) Effects of supervised vs. unsupervised training programs on balance and muscle strength in older adults: a systematic review and meta-analysis. Sports Med 47:2341–2361. https://doi.org/10.1007/s40279-017-0747-6
Rodrigues F, Domingos C, Monteiro D, Morouco P (2022) A review on aging, sarcopenia, falls, and resistance training in community-dwelling older adults. Int J Environ Res Public Health 19(2):874. https://doi.org/10.3390/ijerph19020874
Sherrington C, Whitney JC, Lord SR, Herbert RD, Cumming RG, Close JC (2008) Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc 56: 2234–2243. JGS2014 [pii] https://doi.org/10.1111/j.1532-5415.2008.02014.x
Daly RM, Dalla Via J, Duckham RL, Fraser SF, Helge EW (2019) Exercise for the prevention of osteoporosis in postmenopausal women: an evidence-based guide to the optimal prescription. Braz J Phys Ther 23:170–180. https://doi.org/10.1016/j.bjpt.2018.11.011
Beck BR, Daly RM, Singh MA, Taaffe DR (2016) Exercise and Sports Science Australia (ESSA) position statement on exercise prescription for the prevention and management of osteoporosis. J Sci Med Sport 20:438–445. https://doi.org/10.1016/j.jsams.2016.10.001
Kistler-Fischbacher M, Weeks BK, Beck BR (2021) The effect of exercise intensity on bone in postmenopausal women (part 2): a meta-analysis. Bone 143:115697. https://doi.org/10.1016/j.bone.2020.115697
Gentil P, Arruda A, Souza D et al (2017) Is there any practical application of meta-analytical results in strength training? Front Physiol 8:1. https://doi.org/10.3389/fphys.2017.00001
Zitzmann AL, Shojaa M, Kast S et al (2021) The effect of different training frequency on bone mineral density in older adults. A comparative systematic review and meta-analysis. Bone 154:116230. https://doi.org/10.1016/j.bone.2021.116230
Weineck J (2019) Optimales Training. Spitta-Verlag, Erlangen
Kemmler W, Bebenek M, Kohl M, Von Stengel S (2015) Exercise and fractures in postmenopausal women. Final results of the controlled Erlangen Fitness and Osteoporosis Prevention Study (EFOPS). Osteoporos Int 26:2491–2499. https://doi.org/10.1007/s00198-015-3165-3
Kast S, Shojaa M, Kohl M et al (2022) Effects of different exercise intensity on bone mineral density in adults: a comparative systematic review and meta-analysis. Osteoporos Int. https://doi.org/10.1007/s00198-022-06329-7
Born C, Jakob F, Shojaa M et al (2022) Effects of hormone therapy and exercise on bone mineral density in healthy women - a systematic review and meta-analysis. J Clin Endocrinol Metab. https://doi.org/10.1210/clinem/dgac180
Klotz C, Jakob F, Kohl M et al (2022) Effects of exercise and bisphosphonate-therapy on bone mineral density in healthy women – a systematic review and meta-analysis. Osteo 31(03):184–194. https://doi.org/10.1055/a-1904-5706
Erben RG (2015) Hypothesis: coupling between resorption and formation in cancellous bone remodeling is a mechanically controlled event. Front Endocrinol (Lausanne) 6:82. https://doi.org/10.3389/fendo.2015.00082
Eriksen EF (2010) Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord 11:219–227. https://doi.org/10.1007/s11154-010-9153-1
Shuhart CR, Yeap SS, Anderson PA et al (2019) Executive summary of the 2019 ISCD position development conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, peri-prosthetic and orthopedic bone health, transgender medicine, and pediatrics. J Clin Densitom 22:453–471. https://doi.org/10.1016/j.jocd.2019.07.001
Sterne JA, Sutton AJ, Ioannidis JP et al (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343:d4002. https://doi.org/10.1136/bmj.d4002
Gold EB (2011) The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am 38:425–440. https://doi.org/10.1016/j.ogc.2011.05.002
Acknowledgements
We would like to thank to Dr. Debra and Michael Bemben and Dr. Michel A. Brentano for providing data for the statistical analysis. The present study is part of the German Guideline on Exercise and Fracture Prevention (AWMF No. 183–002) initiated by the Dachverband Osteologie (Osteology umbrella association) Austria/Germany/Suisse. The work was performed in (partial) fulfillment of the requirements for Ramin Mohebbi obtaining the degree Dr. med.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research received no external funding; however, the project S3-Guideline “körperliches Training zur Frakturprophylaxe” was supported by the Elsbeth Bonhoff Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
Not applicable.
Statement of human rights
This article does not cover any studies with human participants with human participants or animals performed by any of the authors.
Conflicts of Interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mohebbi, R., Shojaa, M., Kohl, M. et al. Exercise training and bone mineral density in postmenopausal women: an updated systematic review and meta-analysis of intervention studies with emphasis on potential moderators. Osteoporos Int 34, 1145–1178 (2023). https://doi.org/10.1007/s00198-023-06682-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06682-1