Abstract
Summary
Twelve months following discontinuation of denosumab, the percent decrease in mean bone mineral density (BMD) values at the hip and knee regions were similar between both the denosumab and placebo groups. These findings emphasize the need for additional trials to understand the effect of continued administration of denosumab after subacute spinal cord injury (SCI) to avoid this demineralization.
Objective
To determine changes in BMD 1 year after denosumab was discontinued in participants with subacute SCI who had drug treatment initiated within 90 days post SCI and continued for 1 year.
Methods
Fourteen participants who completed a randomized, double-blinded, placebo-controlled drug trial (parent study: denosumab 60 mg (Prolia, Amgen Inc., n = 8) or placebo (n = 6); administered at baseline, 6, and 12 months) were followed 12 months after the 18 months from baseline primary end point was completed. The BMD of skeletal regions below the SCI at higher risk of fracture was measured [total hip, distal femur epiphysis (DFE), distal femur metaphysis (DFM), and proximal tibia epiphysis (PTE)] by dual energy X-ray absorptiometry.
Results
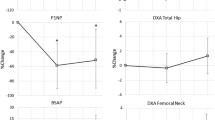
The percent decreases in mean BMD values at all regions of the hip and knee from 18 to 30 months were similar in both the denosumab and placebo groups. However, at 30 months, the absolute values for mean BMD remained significantly higher in the drug treatment than that of the placebo group at the DFM (p = 0.03), DFE (p = 0.04), and PTE (p = 0.05).
Conclusions
In persons with SCI who initiated denosumab treatment during the subacute injury phase and maintained treatment for 1 year, the discontinuation of drug resulted in percent loss of mean BMD similar to that of the placebo group, with absolute mean BMD values at the knee regions at the 12-month follow-up visit significantly higher in the drug treatment than those in the placebo group. These data underscore the need to study continued administration of denosumab after subacute SCI to avoid marked demineralization in the sublesional skeleton upon discontinuation of this agent.


Similar content being viewed by others
References
Lala D, Craven BC, Thabane L, Papaioannou A, Adachi JD, Popovic MR et al (2014) Exploring the determinants of fracture risk among individuals with spinal cord injury. Osteoporos Int 25(1):177–185
Cirnigliaro CM, Myslinski MJ, Asselin P, Hobson JC, Specht A, La Fountaine MF et al (2019) Progressive sublesional bone loss extends into the second decade after spinal cord injury. J Clin Densitom 22(2):185–94
Spungen AM, Bauman WA, Biswas K, Jones KM, Snodgrass AJ, Goetz LL et al (2020) The design of a randomized control trial of exoskeletal-assisted walking in the home and community on quality of life in persons with chronic spinal cord injury. Contemp Clin Trials 96:106102
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361(8):756–765
Yamaguchi Y, Morita T, Kumanogoh A (2020) The therapeutic efficacy of denosumab for the loss of bone mineral density in glucocorticoid-induced osteoporosis: a meta-analysis. Rheumatol Adv Pract 4(1):rkaa008
Oleson CV, Marino RJ, Formal CS, Modlesky CM, Leiby BE (2020) The effect of zoledronic acid on attenuation of bone loss at the hip and knee following acute traumatic spinal cord injury: a randomized-controlled study. Spinal Cord 58:921
Bauman WA, Cirnigliaro CM, La Fountaine MF, Martinez L, Kirshblum SC, Spungen AM (2015) Zoledronic acid administration failed to prevent bone loss at the knee in persons with acute spinal cord injury: an observational cohort study. J Bone Miner Metab 33(4):410–421
Cirnigliaro CM, La Fountaine MF, Parrott JS, Kirshblum SC, McKenna C, Sauer SJ et al (2020) Administration of denosumab preserves bone mineral density at the knee in persons with subacute spinal cord injury: findings from a randomized clinical trial. JBMR Plus 4(8):e10375
McClung MR, Wagman RB, Miller PD, Wang A, Lewiecki EM (2017) Observations following discontinuation of long-term denosumab therapy. Osteoporos Int 28(5):1723–1732
Zanchetta MB, Boailchuk J, Massari F, Silveira F, Bogado C, Zanchetta JR (2018) Significant bone loss after stopping long-term denosumab treatment: a post FREEDOM study. Osteoporos Int 29(1):41–47
Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:863
Garland DE, Adkins RH, Scott M, Singh H, Massih M, Stewart C (2004) Bone loss at the os calcis compared with bone loss at the knee in individuals with spinal cord injury. J Spinal Cord Med 27(3):207–211
Abderhalden L, Weaver FM, Bethel M, Demirtas H, Burns S, Svircev J et al (2017) Dual-energy X-ray absorptiometry and fracture prediction in patients with spinal cord injuries and disorders. Osteoporos Int 28(3):925–934
Frotzler A, Krebs J, Gohring A, Hartmann K, Tesini S, Lippuner K (2020) Osteoporosis in the lower extremities in chronic spinal cord injury. Spinal Cord 58(4):441–448
Lai CH, Chang WH, Chan WP, Peng CW, Shen LK, Chen JJ et al (2010) Effects of functional electrical stimulation cycling exercise on bone mineral density loss in the early stages of spinal cord injury. J Rehabil Med 42(2):150–154
Dudley-Javoroski S, Saha PK, Liang G, Li C, Gao Z, Shields RK (2012) High dose compressive loads attenuate bone mineral loss in humans with spinal cord injury. Osteoporos Int 23(9):2335–2346
Frotzler A, Coupaud S, Perret C, Kakebeeke TH, Hunt KJ, Donaldson Nde N et al (2008) High-volume FES-cycling partially reverses bone loss in people with chronic spinal cord injury. Bone 43(1):169–176
Chen SC, Lai CH, Chan WP, Huang MH, Tsai HW, Chen JJ (2005) Increases in bone mineral density after functional electrical stimulation cycling exercises in spinal cord injured patients. Disabil Rehabil 27(22):1337–1341
Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M et al (2018) Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res 33(2):190–198
Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A (2021) Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med 10(1):152
Tay WL, Tay D (2022) Discontinuing denosumab: can it be done safely? A review of the literature. Endocrinol Metab (Seoul) 37(2):183–194
Zehnder Y, Luthi M, Michel D, Knecht H, Perrelet R, Neto I et al (2004) Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int 15(3):180–189
Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S et al (2012) Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23(1):317–326
Horne AM, Mihov B, Reid IR (2018) Bone loss after romosozumab/denosumab: effects of bisphosphonates. Calcif Tissue Int 103(1):55–61
Kondo H, Okimoto N, Yoshioka T, Akahoshi S, Fuse Y, Ogawa T et al (2020) Zoledronic acid sequential therapy could avoid disadvantages due to the discontinuation of less than 3-year denosumab treatment. J Bone Miner Metab 38(6):894–902
Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM et al (2015) Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet 386(9999):1147–1155
Kendler DL, Bone HG, Massari F, Gielen E, Palacios S, Maddox J et al (2019) Bone mineral density gains with a second 12-month course of romosozumab therapy following placebo or denosumab. Osteoporos Int 30(12):2437–2448
Bubbear JS, Gall A, Middleton FR, Ferguson-Pell M, Swaminathan R, Keen RW (2011) Early treatment with zoledronic acid prevents bone loss at the hip following acute spinal cord injury. Osteoporos Int 22(1):271–279
Goenka S, Sethi S, Pandey N, Joshi M, Jindal R (2018) Effect of early treatment with zoledronic acid on prevention of bone loss in patients with acute spinal cord injury: a randomized controlled trial. Spinal Cord 56(12):1207–1211
Varghese SM, Bobeena RC, Raji T, Tharion J (2016) Effect of zoledronic acid on osteoporosis after chronic spinal cord injury. Crit Rev Phys Rehabil Med 28(1–2):85–93
Schnitzer TJ, Kim K, Marks J, Yeasted R, Simonian N, Chen D (2016) Zoledronic acid treatment after acute spinal cord injury: results of a randomized, placebo-controlled pilot trial. PM R 8:833
Popp AW, Varathan N, Buffat H, Senn C, Perrelet R, Lippuner K (2018) Bone mineral density changes after 1 year of denosumab discontinuation in postmenopausal women with long-term denosumab treatment for osteoporosis. Calcif Tissue Int 103(1):50–54
Asselin P, Cirnigliaro CM, Kornfeld S, Knezevic S, Lackow R, Elliott M et al (2021) Effect of exoskeletal-assisted walking on soft tissue body composition in persons with spinal cord injury. Arch Phys Med Rehabil 102(2):196–202
Ramanujam A, Cirnigliaro CM, Garbarini E, Asselin P, Pilkar R, Forrest GF (2018) Neuromechanical adaptations during a robotic powered exoskeleton assisted walking session. J Spinal Cord Med 41(5):518–528
Knezevic S, Asselin PK, Cirnigliaro CM, Kornfeld S, Emmons RR, Spungen AM (2021) Oxygen uptake during exoskeletal-assisted walking in persons with paraplegia. Arch Phys Med Rehabil 102(2):185–195
Solling AS, Harslof T, Langdahl B (2020) Treatment with zoledronate subsequent to denosumab in osteoporosis: a randomized trial. J Bone Miner Res 35(10):1858–1870
Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC et al (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96(4):972–980
Acknowledgements
The authors wish to thank the James J. Peters VA Medical Center, Bronx, NY, the Department of Veterans Affairs Rehabilitation Research and Development Service, the Kessler Institute for Rehabilitation, and the Kessler Foundation, West Orange, NJ, for their support. The authors would also like to thank Dr. Christopher P. Cardozo for his suggestions with the final version of this manuscript.
Funding
This research was supported by Craig H. Neilson Foundation (#297267) and the VA Rehabilitation Research and Development Service’s National Center for the Medical Consequences of Spinal Cord Injury (#B9212-C, #B2020-C).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Conflict of interest
Christopher M. Cirnigliaro, Michael F. La Fountaine, J. Scott Parrott, Steven C. Kirshblum, Susan J. Sauer, Sue A. Shapses, Isa A. McClure, and William A. Bauman declare that they have no conflicts of interest to report
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cirnigliaro, C.M., La Fountaine, M.F., Parrott, J.S. et al. Loss of lower extremity bone mineral density 1 year after denosumab is discontinued in persons with subacute spinal cord injury. Osteoporos Int 34, 741–748 (2023). https://doi.org/10.1007/s00198-023-06679-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06679-w