Abstract
Summary
Osteoporosis care in men is suboptimal due to low rates of testing and treatment. Applying biomechanical computed tomography (BCT) analysis to existing CT scans, we found a high proportion of men with osteoporosis have never been diagnosed or treated. BCT may improve identification of patients at high risk of fracture.
Purpose
Osteoporosis care in men is suboptimal due to low rates of DXA testing and treatment. Biomechanical computed tomography analysis (BCT) can be applied “opportunistically” to prior hip-containing CT scans to measure femoral bone strength and hip BMD.
Methods
In this retrospective, cross-sectional study, we used BCT in male veterans with existing CT scans to investigate the prevalence of osteoporosis, defined by hip BMD (T-score ≤ − 2.5) or fragile bone strength (≤ 3500 N). 577 men, age ≥ 65 with abdominal/pelvic CTs performed in 2017–2019, were randomly selected for BCT analysis. Clinical data were collected via electronic health records and used with the femoral neck BMD T-score from BCT to estimate 10-year hip fracture risks by FRAX.
Results
Prevalence of osteoporosis by BCT increased with age (13.5% age 65–74; 18.2% age 75–84; 34.3% age ≥ 85), with an estimated overall prevalence of 18.3% for men age ≥ 65. In those with osteoporosis (n = 108/577), only 38.0% (41/108) had a prior DXA and 18.6% (7/108) had received osteoporosis pharmacotherapy. Elevated hip fracture risk by FRAX (≥ 3%) did not fully capture those with fragile bone strength. In a multivariate logistic regression model adjusted for age, BMI, race, and CT location, end stage renal disease (odds ratio 7.4; 95% confidence interval 2.3–23.9), COPD (2.2; 1.2–4.0), and high-dose inhaled corticosteroid use (3.7; 1.2–11.8) were associated with increased odds of having osteoporosis by BCT.
Conclusion
Opportunistic BCT in male veterans provides an additional avenue to identify patients who are at high risk of fractures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of osteoporosis, when defined by either lumbar spine or femoral neck bone mineral density (BMD) T-score ≤ − 2.5 on dual energy X-ray absorptiometry (DXA), is estimated to be 4.3% in US men age 50 and over from the National Health and Nutrition Examination Survey (NHANES) [1]. However, osteoporosis testing by DXA is less commonly performed in older men compared to postmenopausal women. In a large US Veterans Affairs (VA) medical center, only 12% of men age 70 and over received DXA testing compared to 63% of women [2]. Review of Medicare fee-for-service data showed that fewer than 6% of men age 65 and over received DXA testing in the 2 years prior to their fragility fractures [3], and only 5% received testing within 6 months following a new fracture [4]. Additionally, despite higher mortality rates after osteoporotic hip fractures in older men [5, 6], only 15% of US men underwent DXA testing and/or received pharmacology therapy after fragility fractures, compared to 30% of women [7]. Thus, with low rates of DXA testing and subsequent treatment, osteoporosis care in men is currently suboptimal.
There are inherent limitations with DXA, as measured BMD does not reflect important determinants of bone strength, such as bone shape or distribution of cortical vs. trabecular bone [8]. Additionally, BMD as measured by DXA may be confounded by degenerative changes common in older males. Other imaging modalities, such as high-resolution peripheral quantitative computed tomography (HR-pQCT), provide additional information on bone microarchitecture [9], but so far their use remains mostly in clinical research and they do not directly assess the hip or spine, the most relevant sites of osteoporotic fractures. Biomechanical computed tomography analysis (BCT) was developed to perform finite element analysis (FEA) on computed tomography (CT) scans to estimate bone strength in a non-invasive manner. It conducts a virtual “stress test” to determine the force needed to break or fracture the bone of interest [10]. Estimated femoral bone strength by BCT is associated with incident hip fractures in men, independent of BMD [11], and greater reductions in femoral bone strength are seen with aging in comparison to the reductions in femoral neck BMD [12]. Overall, BCT has shown consistent, accurate results in human cadavers [13]. BCT has been validated in clinical studies [10, 11, 14], resulting in its approval by US Food and Drug Administration (FDA) as a class II medical device and coverage by Medicare as a Bone Mass Measurement preventive services benefit [15]. As recommended in the International Society of Clinical Densitometry (ISCD) Official Positions, estimated femoral bone strength by FEA can be used in conjunction with clinical risk factors for consideration of osteoporosis therapy initiation [16].
To advance osteoporosis care in men, we designed this study to evaluate the use of BCT in male veterans age 65 and older who received hip-containing CT scans as part of their medical care. When BCT is applied “opportunistically” to prior hip-containing CT scans performed for unrelated indications, it measures both femoral bone strength using FEA and DXA-equivalent hip BMD (total hip and femoral neck). We hypothesize that osteoporosis is underdiagnosed in older male veterans due to limited BMD testing by DXA. The primary objective of this study was to incorporate opportunistic BCT use to investigate the prevalence of osteoporosis, as defined by either hip BMD (total hip or femoral neck T-score ≤ -2.5) or fragile bone strength (≤ 3500 N), in older men with abdominal or pelvic CT scans. Once we identified these men with osteoporosis from BCT, via electronic health records review, we then determined the proportion of them who had received prior DXA testing or pharmacotherapy treatment for osteoporosis. Next, we examined the clinical parameters associated with osteoporosis diagnosed by BCT to identify specific risk factors that may aid medical providers in deciding which men would be most suitable for opportunistic BCT use in the evaluation of osteoporosis. We then explored whether 10-year hip fracture risks by the Fracture Risk Assessment Tool (FRAX) were able to fully capture these men with fragile bone strength and at high risk of fractures. Finally, we wanted to capture and report our institutional experience with our clinical follow up of participants diagnosed with osteoporosis by BCT.
Materials and methods
This was a retrospective, cross-sectional study conducted at the San Francisco VA Health Care System (SFVAHCS), which encompasses a large academic medical center in an urban city and six community-based clinics across Northern California. The Institutional Review Board of University of California, San Francisco, and San Francisco VA Research and Development Committee approved the study with a waiver of informed consent.
Inclusion and exclusion criteria
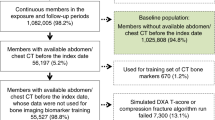
We searched radiology reports of men age 65 and over with abdominal or pelvic CTs performed between September 1, 2017, and September 1, 2019, at SFVAHCS using Illuminate InSight program (Softek Illuminate, Overland Park, KS, USA). To eliminate iatrogenic changes in the femur for BCT analysis, we excluded radiology reports if terms such as “hip arthroplasty,” “hip replacement,” “hip hardware,” “femoral fracture,” “avascular necrosis,” or similar variations of these terms were present. In total, 4209 men had radiology reports that met eligibility criteria (n = 1611 for men age 65–69, n = 1607 for men age 70–74, n = 655 for men age 75–79, n = 398 for men age 80–84, and n = 388 for men age 85 and over). We aimed to have a sample size of 500 with completed BCT analyses. To account for potential un-processable scans to achieve our goal sample size, we intentionally oversampled by randomly selecting CT scans from 625 men in an age-stratified manner (125 men from each half-decade age group) for BCT analysis (Fig. 1). Qualifying CT scans had been acquired on 7 different CT scanner models and from 4 different manufacturers (84% on scanners manufactured by GE Medical Systems. Remaining manufacturers included Philips, Siemens, and Toshiba). Scans were mostly acquired at 120 kVP except for 7% at 140 kVP and 1% at 100 kVP. Slice thickness ranged from 0.62 to 5.0 mm, with majority (65%) performed at a thickness of 2.5 mm.
BCT analysis
After excluding men with duplicate or inadequate scans, CT scans from 617 men were de-identified and sent to a central core laboratory (O.N. Diagnostics, Berkeley, CA, USA) for BCT analysis. More than half (72%) of CT scans were contrasted studies. Details of BCT analysis (VirtuOst 2.1, O.N. Diagnostics) have been previously published [17, 18], with application in both contrasted and non-contrasted CT scans. These studies have shown that the effects of contrast are negligible at the hip, such that BCT analysis can be performed at the hip in individuals receiving contrast [10]. Briefly, a multi-step approach was used to estimate femoral bone strength, defined as the force, in newtons (N), required to virtually break the femur in a sideways-fall configuration. Femoral bone strength was classified using established cut-points: fragile bone strength defined as ≤ 3500 N; low bone strength as > 3500 N but < 5000 N; and normal bone strength as ≥ 5000 N in men [19]. BCT analysis also measured DXA-equivalent BMD at the total hip and femoral neck. Of note, total hip BMD was measured only if CT scan coverage was at least 1 cm distal to the peak of the lesser trochanter. In order to remain consistent with the gender-specific reference database used for DXA reports at SFVAHCS, the young white male NHANES III database was used as the reference standard for BMD T-score calculation. Bone mass was classified using the World Health Organization (WHO) criteria using the lower of the femoral neck and total hip T-scores: osteoporosis defined as T-score ≤ − 2.5, low bone density or osteopenia as T-score > − 2.5 and < − 1.0, and normal bone density as T-score ≥ − 1.0 [20]. For this study, the primary outcome of osteoporosis was defined by either hip BMD (total hip or femoral neck T-score ≤ − 2.5) or fragile bone strength (≤ 3500 N). The decision to define osteoporosis in this manner was based on published literature showing increased sensitivity for incident hip fractures using both hip BMD and skeletal fragility (sensitivity 0.56, 95% confidence interval 0.50–0.62) compared to hip BMD by DXA alone (0.45, 0.37–0.50) or hip BMD by BCT alone (0.45, 0.39–0.52) in men [11].
Clinical data from electronic health records
Investigators blinded from BCT results performed a retrospective review of the VA electronic health records, Computerized Patient Record System (CPRS), to collect relevant clinical parameters. Due to the integrative nature of CPRS, we were able to collect clinical data from other VA medical centers for men who also received medical care outside of SFVAHCS. The following clinical parameters were obtained: demographics, medical conditions, prescriptions, laboratory results, and prior imaging. Demographics included age at time of CT scan, age at death (if deceased at time of chart review), height, weight, BMI, alcohol use, and tobacco use. Medical conditions included those listed in the CPRS problem list by medical providers. Prescriptions included medications of interest ever prescribed within the VA system. Specifically, antiresorptive therapy included oral/intravenous bisphosphonates and denosumab, while anabolic therapy included teriparatide and abaloparatide. Romosozumab was not included as it was only recently approved in 2019. High-dose systemic corticosteroid was defined as an average dose equivalent to prednisone ≥ 5 mg daily. High-dose inhaled corticosteroid was defined using dose cut-points from the National Heart, Lung, and Blood Institute guidelines [21]. Laboratory results included relevant labs performed within 12 months of the CT scans selected for BCT analysis. Of note, urinary calcium and bone turnover markers were collected but not included in the final dataset for statistical analysis as very few men (n < 15) had these measurements available.
10-year hip fracture probability by FRAX
We estimated the hip fracture risk for individual men — the 10-year probability of hip fracture was calculated using the FRAX algorithm for the USA (https://www.sheffield.ac.uk/FRAX/index.aspx). We calculated the FRAX scores using available clinical data obtained from electronic health records and femoral neck BMD T-scores measured by BCT. For those age ≥ 90, we used the age limit of 90, and for those weighing ≥ 125 kg, we used the weight limit of 125 kg imposed by the FRAX algorithm. Due to limitations of retrospective electronic health record review, we could not capture parental history of hip fracture and in turn, may underestimate the true probability of hip fracture.
Clinical follow-up
As part of the IRB approval, we were approved to contact the Primary Care Providers (PCP) of participants found to have osteoporosis on BCT. An IRB approved letter was sent to primary care providers via the electronic medical record system and included BMD, T-score, and femoral strength results from BCT. Diagnosis of osteoporosis by BMD or fragile bone strength was provided to the PCP with recommendation to consider osteoporosis treatment or referral to endocrinology. To determine the clinical impact of diagnosis of osteoporosis by BCT via opportunistic CT scan, we investigated how many veterans were offered osteoporosis evaluation and treatment within the 3 months after BCT result letters were sent to primary care providers.
Statistical methods
We described clinical parameters using mean (standard deviation) or n (%) as appropriate. BMD T-scores and femoral strength were classified based on established cut-points as mentioned above. For our primary objective, we stratified the men into 10-year age groups to investigate the prevalence of osteoporosis across different age groups for our study sample. To estimate the overall prevalence of osteoporosis for male veterans age 65 and over with abdominal/pelvic CT scans performed at SFVAHCS, we first multiplied the prevalence of osteoporosis in each half decade age group (age 65–69, age 70–74, age 75–79, age 80–84, and age 85 and over) with the number of men who had eligible CT scans in each group to estimate the impact of age on prevalence. We then added the number of men across the five age groups and divided this by the total number of men with eligible CT scans to determine the prevalence for the total population. Next, in those men with osteoporosis diagnosed by BCT, descriptive analysis was used to determine the proportion of men who had a prior DXA ever or had received antiresorptive/anabolic therapy.
For the purpose of further characterizing those with osteoporosis newly diagnosed by BCT, we measured the associations between clinical parameters and the dichotomous outcome of osteoporosis diagnosed by BCT in those men without a prior osteoporosis diagnosis or treatment noted in their electronic medical records. We first analyzed proposed clinical predictors using univariate logistic regression. Due to the large number of clinical parameters, we only included predictors with p-value < 0.2 by univariate analysis in backward elimination (p-value < 0.1 to retain). This process yielded a model with five independent risk factors for osteoporosis. Model selection was confirmed using Bayesian information criteria. In the final model, multivariable logistical regression was performed after adjusting for age, BMI, race, and inpatient vs. outpatient CT. Sensitivity analysis was performed separately with models including only hip BMD T-score ≤ − 2.5 or only fragile bone strength as outcomes. Interactions between specific conditions and medications, such as COPD and high-dose systemic corticosteroid use, were also examined. Separate sensitivity analysis was also performed for the entire sample (including men with history of osteoporosis diagnosis or treatment in the electronic medical records) with no significant difference in risk factors found.
Last, we examined the cohort of men with fragile bone strength and normal-to-low BMD (T-score > − 2.5). We again stratified these men into 10-year age groups to investigate the proportion of men with discrepant bone strength and BMD across different age groups. Since current Endocrine Society and National Osteoporosis Foundation clinical guidelines recommend osteoporosis pharmacotherapy initiation in men with BMD T-score between − 1.0 and − 2.5 and 10-year hip fracture risk ≥ 3% by FRAX [22, 23], we calculated the 10-year probability of hip fracture by FRAX for this subgroup of men to determine if FRAX scores alone were able to capture these men at increased fracture risk by fragile bone strength but normal-to-low BMD and whether treatment initiation was indicated. We then separated these men into two groups based on the 10-year probability of hip fracture: high risk (≥ 3%) and low risk (< 3%) for hip fractures. All statistical analyses were performed using Stata version 16 (StataCorp, College Station, TX, USA).
Results
After exclusion of un-processable scans, we obtained BCT results from 557 men (Fig. 1). On average, men were 77.2 years old, white, and overweight (Table 1). Cancer (47.8%), chronic kidney disease (36.6%), and diabetes (30.3%) were the most common medical co-morbidities. As labs were ordered by medical providers for specific indications, only a proportion of men had available testosterone (13.8%), parathyroid hormone (17.9%), and vitamin D (51.7%) measurements within 12 months of the abdominal/pelvic CT scan. The majority of CT scans were performed in the outpatient setting (62.3%), and most commonly for evaluation of malignancy (30.0%).
Prevalence of osteoporosis by BCT
Femoral bone strength and hip BMD decreased with age (Table 2), and these measures were closely correlated (r = 0.92, p < 0.01; data not shown). The prevalence of osteoporosis, defined by either hip BMD (total hip or femoral neck T-score ≤ -2.5) or fragile bone strength (≤ 3500 N) by BCT, increased with age in our sample (Fig. 2). The estimated overall prevalence of osteoporosis, as determined by bone strength and BMD by BCT, in our cohort of male veterans age 65 and over was 18.3% (770/4209). 25.8% of men in our cohort also had a previous major osteoporotic fracture documented on imaging, though whether it was a traumatic or fragility fracture was not able to be determined. The prevalence of osteoporosis as determined by bone strength and BMD by BCT or a history of major osteoporotic fracture was 36.3%. Since many national organizations, including the Endocrine Society and the National Osteoporosis Foundation, recommend BMD testing in men age 70 and over [22, 23], we specifically examined the subgroup of men who met osteoporosis screening criteria by age. In men age 70 and over (n = 443), the prevalence of osteoporosis as determined by BCT was 19.9% using the young white male NHANES III database as reference for BMD T-score. Of note, if the young white female NHANES III database was used instead as reference, the prevalence of osteoporosis was not significantly different at 19.0%. Of note, almost all men (58 out of 64, 93.8%) with hip BMD T-score ≤ − 2.5 had concordant fragile bone strength on BCT. The remaining six men had hip BMD T-scores ≤ − 2.5 and decreased femoral bone strength just above the established cut-points (range: 3530 N − 4080 N; mean 3780 N ± SD 220 N). Men with fragile bone strength and discrepant normal-to-low BMD (T-score > − 2.5) were separately examined and discussed later in this section.
Prior DXA testing or osteoporosis pharmacotherapy
In those men with osteoporosis diagnosed by BCT (n = 108), only 41 men (38.0%) had a prior DXA ever and only 20 men (18.5%) had ever received prior antiresorptive/anabolic therapy. Out of those who received prior DXA testing, 19 men had a prior DXA with T-scores in the osteoporotic range (≤ − 2.5), while 21 men had a prior DXA with T-scores in the low bone density range (< − 1.0 and > − 2.5). DXA testing was performed on average within 3.2 years (± 3.2 years) of hip-containing CT scan used for BCT analysis. Additionally, almost half of men (46 out of 108; 42.6%) meeting osteoporosis diagnosis by BCT had a history of a major osteoporotic fracture on imaging, with 39 (36.1%) of them having documented vertebral compression fractures, although most were not receiving antiresorptive/anabolic therapy. Overall, BCT identified older male veterans with previously undiagnosed or untreated osteoporosis.
Clinical predictors of osteoporosis
To better characterize those men with osteoporosis newly determined by BCT (n = 482), we evaluated the associations between clinical parameters and osteoporosis from BCT in men without prior osteoporosis diagnosis or treatment documented in electronic medical records. In the final multivariate model after adjusting for age, BMI, race, and inpatient vs. outpatient CT, end stage renal disease (ESRD), COPD, exposure to high-dose inhaled corticosteroids, and bone metastasis presence were associated with increased odds of osteoporosis (Table 3). Sensitivity analysis models run separately comparing using only hip BMD T-score ≤ − 2.5 or only fragile bone strength as outcomes showed similar results (not shown). We examined interactions between specific conditions and medications, such as COPD and high-dose systemic corticosteroid use or COPD and high-dose inhaled corticosteroid use, and did not find statistically significant interactions (not shown).
Fragile bone strength and 10-year hip fracture risk
All except one male veteran (57 out of 58) with osteoporosis diagnosed both by fragile bone strength and BMD (T-score ≤ − 2.5) had an elevated 10-year probability of hip fracture by FRAX (Fig. 3). The exception was a 96 year-old man who likely had an underestimation of his true risk due to the imposed age limit of 90 in the FRAX algorithm. However, only a portion (31 out of 44; 70.5%) of men with fragile bone strength and normal-to-low BMD (T-score > − 2.5) demonstrated a 10-year probability of hip fracture ≥ 3% despite being at increased fracture risk by BCT. Therefore, inclusion of a 10-year probability of hip fracture by FRAX captured some, but not all, men with fragile bone strength and increased risk of hip fractures.
10-year hip fracture risk calculation by FRAX captures some, but not all, men with fragile bone strength (≤ 3500 N). High risk was defined as 10-year probability of hip fracture ≥ 3% and low risk as 10-year probability of hip fracture < 3%. BMD, bone mineral density; BCT, biomechanical computed tomography; N, Newton; FRAX, Fracture Risk Assessment Tool
Clinical follow up
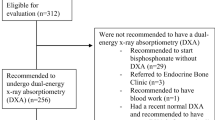
Of the 108 veterans diagnosed with osteoporosis on BCT, 39 patients were deceased or had relocated between the time of the abdominal/pelvis CT scans were obtained and the completion of the study. We sent letters to the primary care providers of the remaining 69 veterans with osteoporosis diagnosed with BCT. Ten additional veterans were deceased by the time result letters were received. Of the 59 remaining veterans, 26 veterans did not have further follow-up regarding osteoporosis, and 5 veterans were already receiving treatment for osteoporosis. Twenty-eight veterans were contacted by their primary care physicians or referred to endocrinology for new osteoporosis evaluation and management as a result of this study (Supplemental Fig. 1).
Discussion
Our findings demonstrated an estimated prevalence of osteoporosis of 18.3% in the US male veterans age 65 and over receiving an abdominal/pelvic CT scan. As expected, the prevalence of osteoporosis increased with age, 19.9% in men age 70 and over (recommended age of BMD testing in men per clinical guidelines) and 34.3% in men age 85 and over. Our observed prevalence of osteoporosis of 18.3%, based on BMD or bone strength, closely matches results from the Focus, Osteoporosis, and CT Utilization Study (FOCUS), which showed osteoporosis by BCT in 18.0% of men age 65 and older receiving care at Kaiser Southern California [11]. Different from previous studies, such as FOCUS, that examined the correlation of DXA and BCT measurements, our study focused on the “opportunistic use” of BCT in men, a population with historically low diagnosis and treatment of osteoporosis. As shown, many of these men with osteoporosis diagnosed by BCT in our study had no prior DXA testing (62.0%) or had prior DXA with BMD T-scores in the low but not osteoporotic bone density range (19.4%). Only a small proportion of all BCT-positive men in our study (18.5%) received prior osteoporosis pharmacotherapy, whereas almost half of them (42.6%) had documented major osteoporotic fractures on prior imaging. Thus, with the opportunistic use of BCT, we were able to capture older men with previously undiagnosed or untreated osteoporosis, with many of them at risk for subsequent fractures.
To aid medical providers to better select men for evaluation of osteoporosis by opportunistic BCT, we identified specific medical conditions that were associated with osteoporosis newly diagnosed by BCT. After accounting for age and other clinical factors, we found that ESRD, COPD, and long-term inhaled corticosteroid use were associated with increased odds of osteoporosis, by either BMD or skeletal fragility. It is well-recognized that patients with these characteristics are at increased risk of fractures [24,25,26]; however, limited studies are available examining these factors in relation to bone strength. We acknowledge that patients with GFR < 15 may often have overlapping osteoporosis and renal osteodystrophy. In our cohort, these men encompass 3% of the cohort and our estimated prevalence of osteoporosis is not appreciably changed with the inclusion of these patients. However, this is a population in which presence of renal osteodystrophy and osteoporosis may contribute to low bone strength and fracture risk and we felt it was important to include in this analysis. Another previous study showed that FEA-derived bone strength from HR-pQCT was altered in women with ESRD but not in men with ESRD [27]. Another study showed FEA-derived radial and tibial failure loads from HR-pQCT were lower in men with both COPD and osteoporosis, although after stratification for BMD, no differences in failure loads were detected in relation to COPD [28]. No previous studies to our knowledge have examined bone strength in men exposed to chronic inhaled corticosteroids. One prior study applying BCT to PET/CT in men with prostate cancer included those with malignant fractures, but did not specifically examine the relation of bone strength and bone metastasis presence [14]. Our study did include men with prostate cancer (16%) and history of androgen deprivation therapy (7%), but sample size was not sufficient for in depth analysis. Overall, our study demonstrated additional factors associated with skeletal fragility that providers could consider to better identify men who may be at increased fracture risk.
With both femoral bone strength and hip BMD measured by BCT, we captured a subset of men with fragile bone strength despite normal-to-low BMD. Studies have shown that estimated femoral strength by FEA is associated with incident hip fractures in men [11, 19, 29] and that after a fragility fracture, the risk of subsequent fracture is increased in patients of all levels of BMD [30]. As discussed earlier, current guidelines recommend therapy initiation in men with low bone density and 10-year hip fracture risk ≥ 3% by FRAX [22, 23]. However, we demonstrated that 10-year hip fracture risk by FRAX alone did not fully capture all men with fragile bone strength — capturing only 70.5% of those with BMD T-scores > − 2.5 and skeletal fragility. Femoral bone strength measured by opportunistic BCT may serve as a valuable addition and used in conjunction with other clinical factors for consideration of pharmacotherapy initiation, as recommended by ISCD [16].
There are several limitations to our retrospective, cross-sectional study. First, medical history and laboratory results collected from electronic health records were provider-driven; thus, there may be men with undiagnosed medical conditions or laboratory abnormalities that could influence our results. Second, medication adherence was difficult to assess using the prescription data collected, so some men may continue to have bone density and strength in the osteoporotic ranges despite adequate pharmacotherapy if adherence is low. Third, parental history of hip fracture was not able to be gathered from retrospective chart review, resulting in possible underestimation of 10-year hip fracture risk by FRAX and may have confounded our results presented in Fig. 3. Last, the generalizability of our results to individuals who have not received CT scans is limited as such individuals may have fewer or different co-morbidities than those men who received CT scans.
Our study is unique in reporting on BCT-based evaluation of osteoporosis in our veterans. This study also has several strengths. First, we were able to collect a comprehensive dataset of clinical parameters due to the well-integrated VA electronic health record system. Second, we included a large cohort of men of diverse racial and ethnic backgrounds in addition to men of advanced age (≥ 85 years old). We attempted to separately examine BCT results in men of Asian (3.8%) and Hispanic (3.1%) backgrounds, but analysis was limited in the setting of sample size. Further studies focusing on BCT use in underrepresented veteran populations should be considered. Third, BCT was applied “opportunistically” to pre-existing hip-containing CT scans obtained for unrelated medical indications. Thus, no additional imaging studies or in-person visits were required. This last strength is important as opportunistic use of BCT may help address certain barriers to BMD testing and ensure timely osteoporosis evaluation.
A novel aspect of this study is that we were also able to show our institutional experience of the clinical impact and change in management as a result of using pre-existing CT scans to diagnose osteoporosis. We found that of the 69 veterans whose providers were contacted, only 7% (n = 5) were already diagnosed and treated for osteoporosis. One of the veterans with osteoporosis diagnosed by BCT was started on treatment after he experienced a hip fracture in the timeframe between the hip-containing CT scan was performed and study completion. We found that 47.5% (n = 28) of veterans whose providers were informed about BCT results were subsequently contacted either by their primary physician or referred to endocrine for new evaluation and treatment of osteoporosis (Supplemental Fig. 1).
In conclusion, our study demonstrated that BCT applied “opportunistically” to hip-containing CT scans identified men age 65 and older with previously undiagnosed or untreated osteoporosis. We found that fragile bone strength was present in older men with normal-to-low BMD and that inclusion of 10-year hip fracture risk by FRAX captured some, but not all, men at increased risk of hip fractures. Men with clinical characteristics, such as history of end-stage renal disease or COPD and exposure to high-dose inhaled steroids, are at higher risk and yet are underdiagnosed for osteoporosis. These clinical parameters, in addition to advanced age of 70 and over, may aid medical providers to better select men in whom to consider proactive or opportunistic use of BCT for evaluation of osteoporosis. Future research directions include prospective studies evaluating systemic BCT use in the diagnosis and treatment of osteoporosis in men.
References
Wright NC, Looker AC, Saag KG et al (2014) The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res 29:2520–2526. https://doi.org/10.1002/jbmr.2269
Narla RR, Hirano LA, Lo SHY et al (2019) Suboptimal osteoporosis evaluation and treatment in older men with and without additional high-risk factors for fractures. J Investig Med 67:743–749. https://doi.org/10.1136/jim-2018-000907
Williams SA, Daigle SG, Weiss R, et al (2020) Characterization of older male patients with a fragility fracture. Arthritis Rheumatol 72:abstr 0533
Hansen D, Pelizzari P, Pyenson B (2021) Medicare cost of osteoporotic fractures — 2021 updated report. Milliman
Haentjens P, Magaziner J, Colón-Emeric CS et al (2010) Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 152:380–390. https://doi.org/10.7326/0003-4819-152-6-201003160-00008
Kannegaard PN, van der Mark S, Eiken P, Abrahamsen B (2010) Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing 39:203–209. https://doi.org/10.1093/ageing/afp221
Balasubramanian A, Tosi LL, Lane JM, et al (2014) Declining rates of osteoporosis management following fragility fractures in the U.S., 2000 through 2009. J Bone Joint Surg Am 96:e52. https://doi.org/10.2106/JBJS.L.01781
Choksi P, Jepsen KJ, Clines GA (2018) The challenges of diagnosing osteoporosis and the limitations of currently available tools. Clin Diabetes Endocrinol 4:12. https://doi.org/10.1186/s40842-018-0062-7
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90:6508–6515. https://doi.org/10.1210/jc.2005-1258
Weber NK, Fidler JL, Keaveny TM et al (2014) Validation of a CT-derived method for osteoporosis screening in IBD patients undergoing contrast-enhanced CT enterography. Am J Gastroenterol 109:401–408. https://doi.org/10.1038/ajg.2013.478
Adams AL, Fischer H, Kopperdahl DL et al (2018) Osteoporosis and hip fracture risk from routine computed tomography scans: the fracture, osteoporosis, and CT utilization study (FOCUS). J Bone Miner Res 33:1291–1301. https://doi.org/10.1002/jbmr.3423
Keaveny TM, Kopperdahl DL, Melton LJ et al (2010) Age-dependence of femoral strength in white women and men. J Bone Miner Res 25:994–1001. https://doi.org/10.1359/jbmr.091033
Johannesdottir F, Thrall E, Muller J et al (2017) Comparison of non-invasive assessments of strength of the proximal femur. Bone 105:93–102. https://doi.org/10.1016/j.bone.2017.07.023
Schwaiger BJ, Kopperdahl DL, Nardo L et al (2017) Vertebral and femoral bone mineral density and bone strength in prostate cancer patients assessed in phantomless PET/CT examinations. Bone 101:62–69. https://doi.org/10.1016/j.bone.2017.04.008
Keaveny TM, Clarke BL, Cosman F et al (2020) Biomechanical computed tomography analysis (BCT) for clinical assessment of osteoporosis. Osteoporos Int 31:1025–1048. https://doi.org/10.1007/s00198-020-05384-2
Shuhart CR, Yeap SS, Anderson PA, et al (2019) Executive summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Peri-prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J Clin Densitom 22:453–471. https://doi.org/10.1016/j.jocd.2019.07.001
Lee DC, Hoffmann PF, Kopperdahl DL, Keaveny TM (2017) Phantomless calibration of CT scans for measurement of BMD and bone strength-Inter-operator reanalysis precision. Bone 103:325–333. https://doi.org/10.1016/j.bone.2017.07.029
Keaveny TM, McClung MR, Genant HK et al (2014) Femoral and vertebral strength improvements in postmenopausal women with osteoporosis treated with denosumab. J Bone Miner Res 29:158–165. https://doi.org/10.1002/jbmr.2024
Kopperdahl DL, Aspelund T, Hoffmann PF et al (2014) Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res 29:570–580. https://doi.org/10.1002/jbmr.2069
Janis KA (2007) Assessment of osteoporosis at the primary health care level. Summary Report of a WHO Scientific Group.
Raissy HH, Kelly HW, Harkins M, Szefler SJ (2013) Inhaled corticosteroids in lung diseases. Am J Respir Crit Care Med 187:798–803. https://doi.org/10.1164/rccm.201210-1853PP
Watts NB, Adler RA, Bilezikian JP et al (2012) Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 97:1802–1822. https://doi.org/10.1210/jc.2011-3045
Cosman F, de Beur SJ, LeBoff MS et al (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381. https://doi.org/10.1007/s00198-014-2794-2
Alem AM, Sherrard DJ, Gillen DL et al (2000) Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 58:396–399. https://doi.org/10.1046/j.1523-1755.2000.00178.x
Biskobing DM (2002) COPD and osteoporosis. Chest 121:609–620
Gonzalez AV, Coulombe J, Ernst P, Suissa S (2018) Long-term use of inhaled corticosteroids in COPD and the risk of fracture. Chest 153:321–328. https://doi.org/10.1016/j.chest.2017.07.002
Trombetti A, Stoermann C, Chevalley T et al (2013) Alterations of bone microstructure and strength in end-stage renal failure. Osteoporos Int 24:1721–1732. https://doi.org/10.1007/s00198-012-2133-4
Romme EAPM, Rutten EPA, Geusens P et al (2013) Bone stiffness and failure load are related with clinical parameters in men with chronic obstructive pulmonary disease. J Bone Miner Res 28:2186–2193. https://doi.org/10.1002/jbmr.1947
Orwoll ES, Marshall LM, Nielson CM et al (2009) Finite element analysis of the proximal femur and hip fracture risk in older men. J Bone Miner Res 24:475–483. https://doi.org/10.1359/jbmr.081201
Conley RB, Adib G, Adler RA et al (2020) Secondary fracture prevention: consensus clinical recommendations from a multistakeholder coalition. J Bone Miner Res 35:36–52. https://doi.org/10.1002/jbmr.3877
Acknowledgements
This study was supported in part by unrestricted investigator-initiated research funds from Radius Health, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
The funding agency had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation or revision of the manuscript. The funding agency reviewed the manuscript prior to submission.
Conflict of interest
DB received research support from Radius for this study and other unrelated projects. ALS received research support from Amgen for other unrelated projects. TMK has been a consultant for Amgen, Agnovos Healthcare, Bone Health Technologies, O.N. Diagnostics, and UCB Pharma, and has equity in O.N. Diagnostics. PT received research support from Radius for this study. JMC does not have any financial or non-financial interests to disclose. PAS does not have any financial or non-financial interests to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
198_2022_6624_MOESM1_ESM.docx
Supplementary file1 Supplemental Figure 1. Clinical follow-up of men diagnosed with osteoporosis by BMD or fragile bone strength on BCT. PCP, Primary Care Physician; BCT, biomechanical computed tomography. (DOCX 32 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Teng, P.F., Chiang, J.M., Schafer, A.L. et al. Prevalence of osteoporosis in older male veterans receiving hip-containing computed tomography scans: opportunistic use of biomechanical computed tomography analysis (BCT). Osteoporos Int 34, 551–561 (2023). https://doi.org/10.1007/s00198-022-06624-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06624-3