Abstract
Summary
Transient insulin resistance seen during puberty is expected to favour body growth, but our results show that increment in insulin resistance even in physiological ranges during puberty might compromise lumbar spine bone mineral density accrual independently of body composition parameters, and therefore adult bone quality might be challenged.
Introduction
Insulin resistance (IR) might have a compromising effect on growing bone, and therefore adult bone quality might be challenged. The aim of the present study was to identify whether increases in IR during puberty contribute to bone mineral characteristics in males independently of body composition parameters.
Methods
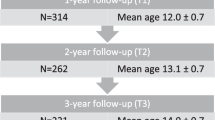
This is a retrospective cohort-based longitudinal observational study. Data from 85 subjects were included. Boys were studied annually during their pubertal years (12 years at baseline) and at follow-up at the age of 18 years. Anthropometry, bone age, fasting blood samples, body composition, total body, and lumbar spine bone mineral characteristics were measured. Insulin resistance was determined by homeostatic model assessment of IR (HOMA-IR). Multiple regression analysis was performed to determine the effect of changes in HOMA-IR during pubertal years as a longitudinal predictor to fixed bone mineral outcome variables at the age of 18 years. All models were adjusted to potential clinically justified confounding variables.
Results
After adjustment to baseline bone indices and body composition-related predictors, the pubertal increment in the HOMA-IR was a negative independent predictor of lumbar spine bone mineral areal density (β = − 0.202, p = 0.005) and lumbar spine bone mineral apparent density (β = − 0.235, p = 0.005) in 18-year-old males.
Conclusions
Pubertal increment in IR has a potential diminishing effect on lumbar spine bone mineral density accrual independently of body composition parameters. Further studies are needed to clarify whether monitoring HOMA-IR during puberty may identify subjects at increased risk of low peak bone mass and possible osteoporosis in the future.
Similar content being viewed by others
References
Shashaj B, Luciano R, Contoli B, Morino GS, Spreghini MR, Rustico C, Sforza RW, Dallapiccola B, Manco M (2016) Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol 53:251–260. https://doi.org/10.1007/s00592-015-0782-4
Jeffery AN, Metcalf BS, Hosking J, Streeter AJ, Voss LD, Wilkin TJ (2012) Age before stage: insulin resistance rises before the onset of puberty: a 9-year longitudinal study (EarlyBird 26). Diabetes Care 35:536–541. https://doi.org/10.2337/dc11-1281
Hannon TS, Janosky J, Arslanian SA (2006) Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res 60:759–763. https://doi.org/10.1203/01.pdr.0000246097.73031.27
Kim SH, Park MJ (2017) Effects of growth hormone on glucose metabolism and insulin resistance in human. Ann Pediatr Endocrinol Metab 22:145–152. https://doi.org/10.6065/apem.2017.22.3.145
Moran A, Jacobs DR Jr, Steinberger J, Cohen P, Hong CP, Prineas R, Sinaiko AR (2002) Association between the insulin resistance of puberty and the insulin-like growth factor-I/growth hormone axis. J Clin Endocrinol Metab 87:4817–4820. https://doi.org/10.1210/jc.2002-020517
Mosca LN, da Silva VN, Goldberg TB (2013) Does excess weight interfere with bone mass accumulation during adolescence? Nutrients 5:2047–2061. https://doi.org/10.3390/nu5062047
Nóbrega da Silva V, Goldberg TBL, Silva CC, Kurokawa CS, Fiorelli LNM, Rizzo ADCB, Corrente JE (2021) Impact of metabolic syndrome and its components on bone remodeling in adolescents. PLoS ONE 16(7):e0253892. https://doi.org/10.1371/journal.pone.0253892
Choo MS, Choi SR, Han JH, Lee SH, Shim YS (2017) Association of insulin resistance with near peak bone mass in the femur and lumbar spine of Korean adults aged 25–35: the Korean National Health and Nutrition Examination Survey 2008–2010. PLoS ONE 12(7):e0177311. https://doi.org/10.1371/journal.pone.0177311
Gracia-Marco L, Ortega FB, Jiménez-Pavón D, Rodríguez G, Castillo MJ, Vicente-Rodríguez G, Moreno LA (2012) Adiposity and bone health in Spanish adolescents. The HELENA study Osteoporos Int 23:937–947. https://doi.org/10.1007/s00198-011-1649-3
Ivuskans A, Lätt E, Mäestu J, Saar M, Purge P, Maasalu K, Jürimäe T, Jürimäe J (2013) Bone mineral density in 11–13-year-old boys: relative importance of the weight status and body composition factors. Rheumatol Int 33:1681–1687. https://doi.org/10.1007/s00296-012-2612-0
Sioen I, Lust E, De Henauw S, Moreno LA, Jiménez-Pavón D (2016) Associations between body composition and bone health in children and adolescents: a systematic review. Calcif Tissue Int 99:557–577. https://doi.org/10.1007/s00223-016-0183-x
Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Brüning JC, Clemens TL (2010) Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142:309–319. https://doi.org/10.1016/j.cell.2010.06.002
Thomas DM, Hards DK, Rogers SD, Ng KW, Best JD (1996) Insulin receptor expression in bone. J Bone Miner Res 11:1312–1320. https://doi.org/10.1002/jbmr.5650110916
Klein GL (2014) Insulin and bone: recent developments. World J Diabetes 5:14–16. https://doi.org/10.4239/wjd.v5.i1.14
Ivaska KK, Heliövaara MK, Ebeling P, Bucci M, Huovinen V, Väänänen HK, Nuutila P, Koistinen HA (2015) The effects of acute hyperinsulinemia on bone metabolism. Endocr Connect 4:155–162. https://doi.org/10.1530/EC-15-0022
Karimi F, Ranjbar Omrani G, Dabbaghmanesh MH (2021) Insulin resistance and bone health in adolescents. Arch Osteoporos 16:66. https://doi.org/10.1007/s11657-021-00917-6
Rønne MS, Heidemann M, Lylloff L, Schou AJ, Tarp J, Bugge A, Laursen JO, Jørgensen NR, Husby S, Wedderkopp N, Mølgaard C (2019) Bone mass development is sensitive to insulin resistance in adolescent boys. Bone 122:1–7. https://doi.org/10.1016/j.bone.2019.02.005
Pollock NK, Bernard PJ, Gutin B, Davis CL, Zhu H, Dong Y (2011) Adolescent obesity, bone mass, and cardiometabolic risk factors. J Pediatr 158(5):727–734. https://doi.org/10.1016/j.jpeds.2010.11.052
Kindler JM, Lobene AJ, Vogel KA, Martin BR, McCabe LD, Peacock M, Warden SJ, McCabe GP, Weaver CM (2019) Adiposity, insulin resistance, and bone mass in children and adolescents. J Clin Endocrinol Metab 104:892–899. https://doi.org/10.1210/jc.2018-00353
Sayers A, Lawlor DA, Sattar N, Tobias JH (2012) The association between insulin levels and cortical bone: findings from a cross-sectional analysis of pQCT parameters in adolescents. J Bone Miner Res 27:610–618. https://doi.org/10.1002/jbmr.1467
do Prado WL, de Piano A, Lazaretti-Castro M, de Mello MT, Stella SG, Tufik S, do Nascimento CM, Oyama LM, Lofrano MC, Tock L, Caranti DA, Dâmaso AR (2009) Relationship between bone mineral density, leptin and insulin concentration in Brazilian obese adolescents. J Bone Miner Metab 27:613–619. https://doi.org/10.1007/s00774-009-0082-6
Dennison EM, Syddall HE, Aihie Sayer A, Craighead S, Phillips DI, Cooper C (2004) Type 2 diabetes mellitus is associated with increased axial bone density in men and women from the Hertfordshire Cohort Study: evidence for an indirect effect of insulin resistance? Diabetologia 47:1963–1968. https://doi.org/10.1007/s00125-004-1560-y
Shin D, Kim S, Kim KH, Lee K, Park SM (2014) Association between insulin resistance and bone mass in men. J Clin Endocrinol Metab 99:988–995. https://doi.org/10.1210/jc.2013-3338
Srikanthan P, Crandall CJ, Miller-Martinez D, Seeman TE, Greendale GA, Binkley N, Karlamangla AS (2014) Insulin resistance and bone strength: findings from the study of midlife in the United States. J Bone Miner Res 29:796–803. https://doi.org/10.1002/jbmr.2083
Lawlor DA, Sattar N, Sayers A, Tobias JH (2012) The association of fasting insulin, glucose, and lipids with bone mass in adolescents: findings from a cross-sectional study. J Clin Endocrinol Metab 97:2068–2076. https://doi.org/10.1210/jc.2011-2721
Lee K (2013) Sex-specific relationships between insulin resistance and bone mineral content in Korean adolescents. J Bone Miner Metab 31:177–182. https://doi.org/10.1007/s00774-012-0396-7
Park SW, Nam GE, Jung DW, Yoon SJ, Han K, Park YG, Choi JS, Lee JE, Sang JE, Yoon YJ, Kim DH (2016) Association of lipid parameters and insulin resistance with bone health in South Korean adolescents. Osteoporos Int 27:635–642. https://doi.org/10.1007/s00198-015-3306-8
Tamme R, Jürimäe J, Mäestu E, Remmel L, Purge P, Mengel E, Tillmann V (2019) Physical activity in puberty is associated with total body and femoral neck bone mineral characteristics in males at 18 years of age. Medicina (Kaunas) 55:203. https://doi.org/10.3390/medicina55050203
Tanner JM, Whitehouse RH, Marubini E, Resele LF (1976) The adolescent growth spurt of boys and girls of the Harpenden growth study. Ann Hum Biol 3:109–126. https://doi.org/10.1080/03014467600001231
Matsudo SMM, Matsudo VKR (1994) Self-assessment and physician assessment of sexual maturation in Brazilian boys and girls: concordance and reproducibility. Am J Hum Biol 6:451–455. https://doi.org/10.1002/ajhb.1310060406
Greulich WW, Pyle SI (1959) Radiographic atlas of skeletal development of hand and wrist, 2nd edn. Stanford University Press, Stanford
Katzman DK, Bachrach LK, Carter DR, Marcus R (1991) Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab 73:1332–1339. https://doi.org/10.1210/jcem-73-6-1332
Wallace TM, Levy JC, Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495. https://doi.org/10.2337/diacare.27.6.1487
Tamme R, Jürimäe J, Mäestu E, Remmel L, Purge P, Mengel E, Tillmann V (2019) Association of serum testosterone at 12 years with a subsequent increase in bone mineral apparent density at 18 years: a longitudinal study of boys in puberty. Horm Res Paediatr 91:400–405. https://doi.org/10.1159/000502606
Welten M, de Kroon MLA, Renders CM, Steyerberg EW, Raat H, Twisk JWR, Heymans MW (2018) Repeatedly measured predictors: a comparison of methods for prediction modeling. Diagn Progn Res 2:5. https://doi.org/10.1186/s41512-018-0024-7
Kanis JA, Cooper C, Rizzoli R, Reginster JY; Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF) (2019) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30:3–44. https://doi.org/10.1007/s00198-018-4704-5
Kraenzlin ME, Meier C (2011) Parathyroid hormone analogues in the treatment of osteoporosis. Nat Rev Endocrinol 7:647–656. https://doi.org/10.1038/nrendo.2011.108
Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3(3):S131–S139. https://doi.org/10.2215/CJN.04151206
Mora S, Goodman WG, Loro ML, Roe TF, Sayre J, Gilsanz V (1994) Age-related changes in cortical and cancellous vertebral bone density in girls: assessment with quantitative CT. AJR Am J Roentgenol 162:405–409. https://doi.org/10.2214/ajr.162.2.8310936
Mengel E, Tillmann V, Remmel L, Kool P, Purge P, Lätt E, Jürimäe J (2017) Extensive BMI gain in puberty is associated with lower increments in bone mineral density in Estonian boys with overweight and obesity: a 3-year longitudinal study. Calcif Tissue Int 101:174–181. https://doi.org/10.1007/s00223-017-0273-4
Mihalopoulos NL, Holubkov R, Young P, Dai S, Labarthe DR (2010) Expected changes in clinical measures of adiposity during puberty. J Adolesc Health 47:360–366. https://doi.org/10.1016/j.jadohealth.2010.03.019
Torres-Costoso A, Pozuelo-Carrascosa DP, Álvarez-Bueno C, Ferri-Morales A, Miota Ibarra J, Notario-Pacheco B, Martínez-Vizcaíno V (2017) Insulin and bone health in young adults: the mediator role of lean mass. PLoS ONE 12:e0173874. https://doi.org/10.1371/journal.pone.0173874
Paquin J, Lagacé JC, Brochu M, Dionne IJ (2021) Exercising for insulin sensitivity - is there a mechanistic relationship with quantitative changes in skeletal muscle mass? Front Physiol 12:656909. https://doi.org/10.3389/fphys.2021.656909
Raygor V, Abbasi F, Lazzeroni LC, Kim S, Ingelsson E, Reaven GM, Knowles JW (2019) Impact of race/ethnicity on insulin resistance and hypertriglyceridaemia. Diab Vasc Dis Res 16:153–159. https://doi.org/10.1177/1479164118813890
Funding
This study was funded by the Estonian Research Council grant (PRG 1120 and PRG 1428).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mengel, E., Tamme, R., Remmel, L. et al. Pubertal increment in insulin resistance is negatively related to lumbar bone mineral density in 18-year-old males. Osteoporos Int 34, 161–170 (2023). https://doi.org/10.1007/s00198-022-06591-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06591-9