Abstract
Summary
In this randomized, controlled trial, sequential therapy with once-weekly subcutaneous injection of teriparatide for 72 weeks, followed by alendronate for 48 weeks resulted in a significantly lower incidence of morphometric vertebral fracture than monotherapy with alendronate for 120 weeks in women with osteoporosis at high risk of fracture.
Purpose
To determine whether the anti-fracture efficacy of sequential therapy with teriparatide, followed by alendronate is superior to that of monotherapy with alendronate, a prospective, randomized, open-label, blinded-endpoint trial was performed.
Methods
Japanese women aged at least 75 years were eligible for the study, if they had primary osteoporosis and if they were at high risk of fracture. Patients were randomly assigned (1:1) to receive the sequential therapy (once-weekly subcutaneous injection of teriparatide 56.5 μg for 72 weeks, followed by alendronate for 48 weeks) or monotherapy with alendronate for 120 weeks. The primary endpoint in the final analysis was the incidence of morphometric vertebral fracture during the 120-week follow-up period.
Results
Between October 2014 and June 2020, 505 patients in the sequential therapy group and 506 in the monotherapy group were enrolled. Of these, 489 and 496, respectively, were included in the main analysis. The incidence of morphometric vertebral fracture during the 120-week follow-up period in the sequential therapy group (64 per 627.5 person-years, annual incidence rate 0.1020) was significantly lower than that in the monotherapy group (126 per 844.2 person-years, annual incidence rate 0.1492), with a rate ratio of 0.69 (95% confidence interval 0.54 to 0.88, P < 0.01). After 72 weeks, no patient had a severe adverse event that was considered related to the study drug.
Conclusion
Once-weekly injection of teriparatide, followed by alendronate resulted in a significantly lower incidence of morphometric vertebral fracture than alendronate monotherapy in women with osteoporosis who were at high risk of fracture.
Trial registration number, date of registration
jRCTs031180235 and UMIN000015573, March 12, 2019
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the diagnosis of osteoporosis relies heavily on the presence of low bone mineral density (BMD), clinical factors such as age, sex, and prior fragility fracture also have a pivotal role in assessing the risk of fracture [1,2,3]. For example, in elderly women with osteoporosis, the site of an existing fracture and whether its history is recent affect the risk of subsequent fracture. Patients with so-called “severe osteoporosis” should be treated with a potent anti-osteoporosis agent. However, there is no consensus about the long-term treatment strategy for severe osteoporosis.
Current treatment guidelines recommend once-daily subcutaneous injection of teriparatide for patients at high risk of fracture [3, 4]. Furthermore, once-weekly subcutaneous injection of teriparatide has been approved in Japan based on the results of a randomized, placebo-controlled trial [5]. This new regimen requires less frequent administration and may not cause deleterious changes in the cortical microarchitecture compared with the once-daily regimen [6]. These results suggest that treatment for patients with severe osteoporosis should be started with once-weekly injection of teriparatide. However, the use of teriparatide is limited to 24 months, irrespective of the regimen. As a result, teriparatide must be switched to another medication if patients have received it for the approved period because of the concern that BMD may decrease after terminating teriparatide treatment [7, 8].
In a follow-up observational study of the Japanese placebo-controlled trial mentioned above, patients who received bisphosphonate following teriparatide achieved a further increase in BMD [9]. A randomized, controlled trial also indicated that the sequential therapy with full-length parathyroid hormone, followed by alendronate significantly increased areal BMD at the lumbar spine, compared with parathyroid hormone therapy, followed by placebo or monotherapy with alendronate alone [10]. These results suggest that alendronate, one of the most commonly used anti-resorptive agents, is a suitable candidate drug for patients at high risk of osteoporotic fracture.
However, no randomized, controlled trial has evaluated the anti-fracture efficacy of sequential therapy. In this context, the Adequate Treatment of Osteoporosis (A-TOP) research group conducted the Japanese Osteoporosis Intervention Trial-05 (JOINT-05) to compare the efficacy and safety of the sequential therapy (once-weekly injection of teriparatide for 72 weeks, followed by alendronate for 48 weeks) and monotherapy (alendronate for 120 weeks) in women at high risk of fracture. In the first part of the study up to 72 weeks, it was found that teriparatide was superior to alendronate in reducing the incidence of morphometric vertebral fracture [11]. In the second part from 72 to 120 weeks, teriparatide was switched to alendronate in the sequential therapy group, and the hypothesis that the anti-fracture efficacy of sequential therapy throughout 120 weeks is superior to that of monotherapy was tested. In this paper, the final results are reported.
Methods
Study design
JOINT-05 was a prospective, randomized, open-label, blinded-endpoint trial conducted between October 2014 and June 2020 at 113 institutions in Japan. The protocol was approved by the certified review board of Toranomon Hospital and the central ethics committee of the A-TOP research group. The study was conducted in accordance with the Declaration of Helsinki and the Clinical Trials Act of the Japanese Ministry of Health, Labour, and Welfare. All patients provided written, informed consent. The study is registered with the Japan Registry of Clinical Trials (number, jRCTs031180235) and the University Hospital Medical Information Network-Clinical Trials Registry (number, UMIN000015573).
Study population
The design of the JOINT-05 has been reported previously [11, 12]. In brief, Japanese women aged at least 75 years were eligible for the study, if they had primary osteoporosis and if they were at high risk of fracture. Primary osteoporosis was diagnosed, according to the revised Diagnostic Criteria for Primary Osteoporosis of the Japanese Society for Bone and Mineral Research [13]. Specifically, primary osteoporosis was diagnosed in women who had no disease that causes low bone mineral density other than osteoporosis, who had no secondary osteoporosis and have a fragility fracture or a BMD of < 70% of the young adult mean. Patients at high risk of fracture were defined as those who had any of the following: BMD less than 60% of young adult mean (at the lumbar spine, proximal femur, radius, and second metacarpal bone) or less than − 3.3 standard deviations (SDs); at least 2 vertebral fractures in the area from the fourth thoracic vertebra (Th4) to the fourth lumbar vertebra (L4); a grade 3 prevalent fracture; or past hip fracture.
Eligibility criteria
Patients were eligible for the study, if they were:
-
1.
diagnosed with primary osteoporosis, according to the revised 2012 Diagnostic Criteria for Primary Osteoporosis of the Japanese Society for Bone and Mineral Research [13];
-
2.
women at least 75 years of age when giving informed consent;
-
3.
could walk by themselves (walk alone, with a cane, or with a walker); and
-
4.
at high risk of fracture (i.e., BMD less than 60% of young adult mean or less than − 3.3 SDs, at least 2 vertebral fractures in the area from Th4 to L4, or a grade 3 prevalent fracture or past fracture).
Patients were excluded from the study, if they had:
-
1.
secondary osteoporosis;
-
2.
diagnosis of a disease other than osteoporosis that causes bone loss;
-
3.
diagnosis of a disease that affects the strength of the vertebral bodies;
-
4.
history of hypersensitivity such as bronchial asthma or rash;
-
5.
contraindication to any of the study drugs used;
-
6.
serious renal, hepatic, or cardiac disease;
-
7.
been hospitalized; or
-
8.
history of treatment with teriparatide.
Study treatments
Patients were randomly assigned in a 1:1 ratio to receive the sequential therapy (teriparatide 56.5 μg for 72 weeks, followed by alendronate for 48 weeks) or monotherapy with alendronate for 120 weeks. Teriparatide was administered subcutaneously once weekly. Alendronate was administered using the following formulations: 5 mg tablet (orally administered once daily), 35 mg tablet or jelly (orally administered once weekly), or 900 μg infusion bag (administered intravenously once every 4 weeks). In the sequential therapy group, 0% received the 5 mg tablet, 54.4% received the 35 mg tablet, 14.5% received the 35 mg oral jelly, and 31.1% received the 900 μg infusion bag. In the monotherapy group, 0.6% received the 5 mg tablet, 45.9% received the 35 mg tablet, 16.4% received the 35 mg oral jelly, and 37.1% received the 900 µg infusion bag. Medication compliance was monitored by recording the dates teriparatide prescriptions were issued and verifying alendronate compliance by the physician in charge approximately every 12 weeks.
To ensure balanced treatment groups at enrollment, subjects were randomly allocated by a web-based computerized system with the modified minimization method [14] adjusted for the following factors which could affect the assessment of treatment efficacy: age (75–79 vs. ≥ 80 years), BMD (< 60% vs. ≥ 60% of young adult mean), number of prevalent vertebral fractures (0–1 vs. ≥ 2), presence or absence of prevalent vertebral fracture of grade 3, presence or absence of history of hip fracture, and study institution. The algorithm for random allocation was concealed from the study personnel.
Outcome measures
The thoracic and lumbar vertebrae were imaged in two directions at 0 (baseline), 24, 48, 72, and 120 weeks. For the assessment of prevalent vertebral fracture, anteroposterior and lateral radiographs of the thoracic and lumbar spine were examined by the investigators. The grade of vertebral fracture from Th4 to L4 was assessed using the semiquantitative (SQ) technique to classify fractures as mild (grade 1: 20% to 25% reduction in vertebral height/10% to 20% reduction in vertebral area), moderate (grade 2: 25% to 40% reduction in vertebral height/20% to 40% reduction in vertebral area), or severe (grade 3: ≥ 40% decrease in vertebral height/ ≥ 40% reduction in vertebral area) [15]. Fractures were considered to have progressed if the SQ grade changed from grade 1 to grade 2 or from grade 2 to grade 3. These assessments were reviewed centrally by one evaluator from the fracture assessment committee blinded to the assigned treatment. The committee member also adjudicated the presence or absence of a new vertebral fracture by comparing radiographs of Th4 to L4 between baseline and post-treatment. After the X-ray films were collected, two evaluators blinded to the assigned treatment reviewed the films independently, according to the SQ technique mentioned above. If inconsistencies arose between the evaluators, three evaluators reviewed the films simultaneously. The presence or absence of other fractures, such as non-vertebral fractures (fractures at any sites other than the vertebrae) and clinical fractures, was assessed by the investigators. Thereafter, three evaluators from the fracture assessment committee reviewed the assessment made by the investigators using the collected X-ray films.
BMD at the lumbar spine, proximal femur, radius, and second metacarpal bone was measured in each institution at 0, 24, 48, 72, and 120 weeks by dual-energy X-ray absorptiometry. No cross-calibration of machines was performed across institutions. Blood samples were obtained at 0, 12, 24, 48, 72, and 120 weeks to measure the serum levels of osteocalcin, procollagen type I amino-terminal propeptide (P1NP), and tartrate-resistant acid phosphatase 5b (TRACP-5b). The inter-assay coefficients of variation were 4.48–8.64% for osteocalcin, 1.09–1.74% for P1NP, and 2.5–4.4% for TRACP-5b. LSI Medience Corporation (Tokyo, Japan) analyzed the levels of osteocalcin and P1NP using a fluorometric enzyme immunoassay and an electrochemiluminescence immunoassay, respectively. SB Bioscience Co., Ltd. (Tokyo, Japan) analyzed TRACP-5b levels using an enzyme immunoassay.
The primary endpoint in the second part of the study was the incidence of morphometric vertebral fracture from 0 to 120 weeks. The accumulation of person-years at risk started at the randomization of each patient and ended at the date of the last visit, lost to follow-up, or death. One of the secondary endpoints in the second part was the incidence of morphometric vertebral fracture from 72 to 120 weeks. Other secondary endpoints included the following from 0 to 120 weeks: incidence of any fracture, clinical vertebral fracture, non-vertebral fracture, and fracture at specific skeletal sites; and progression of vertebral fracture.
Statistical considerations
Prior to the start of the study, it was assumed that the annualized incidence of vertebral fracture in the alendronate group would be 0.112 and that the hazard ratio of teriparatide relative to alendronate over 72 weeks would be 0.5 [12]. We assumed the annualized incidence of non-vertebral fracture would be 0.016 and the non-inferiority margin was set at a hazard ratio of 1.96 based on effect retention as calculated by comparing the hazard ratio of alendronate with placebo. Based on these assumptions, a sample size of 500 patients for each group was calculated to be required in order to detect the difference in the incidence of morphometric vertebral fracture in the first part (from 0 to 72 weeks) between the treatment groups with a power of 0.80 and a significance level of 0.05 [11, 12]. In the final analysis of vertebral fracture at 120 weeks, the power was estimated to be 0.99 given that the analysis population consisted of 778 patients, and the follow-up periods were longer than those in the primary analysis.
Efficacy outcomes were analyzed in the full analysis set, which consisted of randomly assigned patients who received at least one dose of the study medication and had at least one evaluable radiograph after randomization. In the analyses of the fractures, a multivariable Poisson regression model was fit to calculate the rate ratios of sequential therapy to monotherapy and their 95% confidence intervals (CIs). This regression model included the minimization factors for random allocation as covariates. Missing data on radiographs of Th4 to L4 were not imputed. For the incidences of morphometric vertebral fracture, any fracture, and clinical vertebral fracture, as well as the progression of vertebral fracture, the hypothesis that the efficacy of sequential therapy is superior to that of monotherapy was tested. For the incidence of non-vertebral fractures, the hypothesis that the efficacy of sequential therapy is not inferior to that of monotherapy, defined as the upper limit of the 95% CI for the rate ratio less than 1.96, was tested.
Safety outcomes were analyzed descriptively by treatment group in the safety analysis set, which consisted of all patients who entered the second 48-week treatment period. Because the proportion of patients who had any adverse event up to 72 weeks in the first part was analyzed [11], the focus in this report was on the adverse events that occurred after 72 weeks. Adverse events were coded, according to the system organ class of the Medical Dictionary for Regulatory Activities.
All data were analyzed with the use of SAS® software version 9.4 (SAS Institute, Cary, NC). All reported P values are 2-tailed without adjustment for multiplicity.
Results
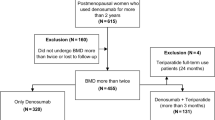
A total of 1011 patients (505 in the sequential therapy group and 506 in the monotherapy group) were enrolled in the study (Fig. 1). Of these, 489 and 496 patients in the sequential therapy and monotherapy groups, respectively, were included in the full analysis set (Online Resource 1). At 72 weeks, 251 and 357 patients, respectively, remained in the study. These patients were included in the safety analysis set. As shown in Fig. 1, 205 out of the 251 patients in the sequential therapy group switch to alendronate, and 348 out of 357 patients in the monotherapy group continued alendronate beyond 80 weeks. In addition, 265 and 184 patients, respectively, discontinued the follow-up from 72 to 120 weeks. The main reasons for discontinuation were the patient’s wish and safety reasons.
Baseline characteristics were well balanced between the two groups (Table 1). The mean (SD) age was 81.4 (4.5) years in the sequential therapy group and 81.5 (4.7) years in the monotherapy group. Two-thirds of the patients in each group had prevalent vertebral fractures, and approximately 40% of the patients in each group had at least 2 prevalent vertebral fractures and at least 1 grade 3 prevalent vertebral fracture.
In the analysis of fracture risk (Table 2), the incidence of morphometric vertebral fracture from 0 to 120 weeks was significantly lower in the sequential therapy group (64 per 627.5 person-years, annual incidence rate 0.1020) than in the monotherapy group (126 per 844.2 person-years, annual incidence rate 0.1492), with a rate ratio of 0.69 (95% CI 0.54 to 0.88, P < 0.01). The incidence of morphometric vertebral fracture from 72 to 120 weeks was also significantly lower in the sequential therapy group (annual incidence rate 0.0376) than in the monotherapy group (annual incidence rate 0.1008), with a rate ratio of 0.41 (95% CI 0.24 to 0.71, P < 0.01).
The incidence of non-vertebral fracture during 120 weeks in the sequential therapy group was 23 per 678.3 person-years (annual incidence rate 0.0339), whereas that in the monotherapy group was 25 per 891.3 person-years (annual incidence rate 0.0280), with a rate ratio of 1.27 (95% CI 0.84 to 1.93, P = 0.04 for non-inferiority). Analyses of the other endpoints did not show significant treatment effects.
Similar elevations in mean BMD (T-score) at the lumbar spine from 0 to 72 weeks were seen in the two groups (Fig. 2). However, the increase in BMD after 72 weeks was numerically greater in the sequential therapy group, although the difference between the groups at 120 weeks was not significant (P = 0.09). Serum levels of osteocalcin, P1NP, and TRACP-5b decreased in the sequential therapy group after 72 weeks, whereas they remained approximately constant in the monotherapy group. As a result, their serum levels at 120 weeks were similar in the two groups.
Changes in bone mineral density at the lumbar spine and bone turnover markers over 120 weeks by treatment group. Percent changes in bone mineral density were 9.0% (n = 192), 12.3% (n = 165), 15.0% (n = 151), and 20.2% (n = 129) at each time point in the sequential group and 7.2% (n = 228), 10.7% (n = 220), 13.5% (n = 208), and 14.8% (n = 177) at each time point in the monotherapy group. Percent changes in osteocalcin were 54.2% (n = 364), 50.1% (n = 338), 35.2% (n = 288), 33.1% (n = 263), and − 28.9% (n = 221) at each time point in the sequential group and − 8.5% (n = 424), − 19.6% (n = 416), − 25.9% (n = 392), − 28.7% (n = 362), and − 28.3% (n = 308) at each time point in the monotherapy group. Percent changes in P1NP were 21.9% (n = 364), 18.7% (n = 338), 7.9% (n = 288), 7.9% (n = 263), and − 42.5% (n = 221) at each time point in the sequential group and − 30.3% (n = 424), − 38.7% (n = 416), − 39.9% (n = 392), − 42.4% (n = 362), and − 37.3% (n = 308) at each time point in the monotherapy group. Percent changes in TRACP-5b were − 2.1% (n = 363), − 5.3% (n = 338), − -11.8% (n = 288), − 10.4% (n = 263), and − 24.3% (n = 221) at each time point in the sequential group and − 23.1% (n = 425), − 26.3% (n = 416), − 28.3% (n = 392), − 27.0% (n = 362), and − 21.6% (n = 308) at each time point in the monotherapy group. Abbreviations: BMD, bone mineral density; P1NP, procollagen type I amino-terminal propeptide; TRACP-5b, tartrate-resistant acid phosphatase 5b. The term “teriparatide to alendronate” means the sequential therapy group, whereas “alendronate to alendronate” means the monotherapy group
The treatment effects of sequential therapy on the incidence of morphometric vertebral fracture were generally consistent across various subgroups (Fig. 3). However, sequential therapy showed a good effect in patients with grade 3 fracture, whereas monotherapy showed it in those with grade 1–2 fracture (P = 0.05 for interaction).
In both groups, adverse events were most frequently reported in the following system organ classes after 72 weeks: infections and infestations, gastrointestinal disorders, musculoskeletal and connective tissue disorders, and general disorders and administration site conditions (Table 3). The proportion of patients who had adverse events classified into these system organ classes was numerically lower in the sequential therapy group than in the monotherapy group. After 72 weeks, no patient had a severe adverse event that was considered related to the study drug by the investigator.
Discussion
In the final analysis of JOINT-05, the incidence of morphometric vertebral fracture from 0 to 120 weeks was significantly lower in the sequential therapy group than in the monotherapy group. Furthermore, the effect of sequential therapy on the reduction in non-vertebral fractures was not inferior to that of monotherapy. Although a previous randomized, controlled trial showed the superiority of sequential therapy with parathyroid hormone, followed by alendronate over monotherapy with alendronate in increasing BMD [10], it did not assess their effects on the rate of fracture. To the best of our knowledge, the present trial is the first head-to-head comparison of the anti-fracture efficacy of sequential therapy.
Alendronate was used as an anti-resorptive agent following teriparatide, but several studies suggested that denosumab might be another candidate [16,17,18,19]. Of these, two studies indicated that the increase in BMD at the lumbar spine was significantly greater in the teriparatide-to-denosumab group than in the teriparatide-to-bisphosphonate group [17, 18]. However, denosumab has to be subcutaneously injected. In contrast, alendronate can be administered orally, which is a more convenient route of administration for long-term use. In addition, the reductions in fracture risk were similar between denosumab and bisphosphonates, although their effects on BMD seemed to be different [20]. Thus, alendronate is considered to be a suitable sequential therapy, following teriparatide for patients at high risk of osteoporotic fracture.
In the second part of JOINT-05, all patients received alendronate, but the incidence of morphometric vertebral fracture from 72 to 120 weeks was significantly lower in the sequential therapy group. Furthermore, the increase in lumbar BMD after 72 weeks was numerically greater in the sequential therapy group. These results are consistent with those obtained from the previous studies that assessed the effects of anti-resorptive treatment following teriparatide on BMD [10, 16, 21]. Of these, two studies showed rapid reduction in bone resorption marker levels after switching to anti-resorptive treatment accompanied by relatively slower reduction in bone formation marker levels [16, 22]. These results may explain the mechanism underlying the favorable effects of sequential therapy.
Subgroup analyses have suggested the possible superiority of sequential therapy in patients with grade 3 prevalent vertebral fracture. These results are consistent with those obtained from the analysis of the first part [11]. Currently, teriparatide is strongly recommended for patients at very high risk of fracture [4, 22]. However, its use is limited to 24 months, and there is no consensus about the long-term treatment strategy for these patients. The present results suggest that teriparatide, followed by alendronate may be especially effective in these patients.
In the safety assessments, common adverse events reported in the second part were similar to those reported in the first part [11]. No adverse event indicating a new safety concern was observed. Although the proportion of patients who had any adverse event was lower in the second part than in the first part regardless of the assigned treatment, it may be due to the difference in treatment period between the first (72 weeks) and second (48 weeks) parts.
The overall safety profile of bisphosphonates is good [3]. Gastrointestinal disorders were frequently reported in the present study. These disorders are known to be associated with oral bisphosphonates, and proper instructions, such as remaining in the upright position for at least 30 min after intake, can reduce their frequency [23]. Musculoskeletal and connective tissue disorders were also frequently reported. Of these, myalgia, arthralgia, and bone pain are considered acute-phase reactions and generally transient [23].
Some limitations should be mentioned. First, the optimal treatment duration of alendronate following teriparatide could not be assessed because the treatment period of the second part was 48 weeks. However, the Task Force of the American Society for Bone and Mineral Research recommends 10-year treatment with oral bisphosphonate or 6-year treatment with intravenous bisphosphonate accompanied by periodic evaluation of fracture risk for women at high risk of fracture [24]. Therefore, the optimal duration may be longer than 6 or 10 years and should be assessed in future observational studies. Second, due to the large number of patients lost to follow-up, the final sample size was not large enough to detect differences between the treatment groups in the incidences of any fracture, the incidence of clinical vertebral fracture, and the progression of vertebral fracture. A larger portion of subjects discontinued the study drugs in the sequential group than in the monotherapy group. This is most likely due to higher drug prices and the greater time and effort required attending hospital visits. Although it can be speculated that pharmacological effects also resulted in the large portion of subjects discontinuing the study drugs, no patient had a severe adverse event that was considered related to the study drug after 72 weeks.
Third, BMD was measured in each institution without specifying the analytical method. Thus, the changes in BMD should be interpreted cautiously.
In conclusion, sequential therapy with once-weekly subcutaneous injection of teriparatide, followed by alendronate was associated with a significantly greater reduction in the incidence of morphometric vertebral fracture than monotherapy with alendronate in women with primary osteoporosis who were at high risk of fracture. We consider that alendronate is a suitable successor to teriparatide.
References
Delmas PD, Rizzoli R, Cooper C, Reginster JY (2005) Treatment of patients with postmenopausal osteoporosis is worthwhile. The position of the International Osteoporosis Foundation. Osteoporos Int 16:1–5
Kanis JA, Borgstrom F, De Laet C et al (2005) Assessment of fracture risk. Osteoporos Int 16:581–589
Kanis JA, Cooper C, Rizzoli R, Reginster JY (2019) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30:3–44
Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D (2019) Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 104:1595–1622
Nakamura T, Sugimoto T, Nakano T et al (2012) Randomized teriparatide [human parathyroid hormone (PTH) 1–34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab 97:3097–3106
Yamane H, Takakura A, Shimadzu Y et al (2017) Acute development of cortical porosity and endosteal naïve bone formation from the daily but not weekly short-term administration of PTH in rabbit. PLoS One 12(4):e0175329. https://doi.org/10.1371/journal.pone.0175329
Eastell R, Nickelsen T, Marin F et al (2009) Sequential treatment of severe postmenopausal osteoporosis after teriparatide: final results of the randomized, controlled European Study of Forsteo (EUROFORS). J Bone Miner Res 24:726–736
Adami S, San Martin J, Muñoz-Torres M et al (2008) Effect of raloxifene after recombinant teriparatide [hPTH(1–34)] treatment in postmenopausal women with osteoporosis. Osteoporos Int 19:87–94
Sugimoto T, Shiraki M, Nakano T et al (2013) Vertebral fracture risk after once-weekly teriparatide injections: follow-up study of Teriparatide Once-Weekly Efficacy Research (TOWER) trial. Curr Med Res Opin 29:195–203
Black DM, Bilezikian JP, Ensrud KE et al (2005) One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 353:555–565
Hagino H, Sugimoto T, Tanaka S et al (2021) A randomized, controlled trial of once-weekly teriparatide injection versus alendronate in patients at high risk of osteoporotic fracture: primary results of the Japanese Osteoporosis Intervention Trial-05. Osteoporos Int 32:2301–2311
Tanaka S, Mori S, Hagino H, Sugimoto T (2020) Design of a randomized trial of teriparatide followed by alendronate: Japanese Osteoporosis Intervention Trial-05 (JOINT-05). J Bone Miner Metab 38:412–417
Soen S, Fukunaga M, Sugimoto T et al (2013) Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab 31:247–257
Scott NW, McPherson GC, Ramsay CR, Campbell MK (2002) The method of minimization for allocation to clinical trials: a review. Control Clin Trials 23:662–674
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Leder BZ, Tsai JN, Uihlein AV et al (2015) Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet 386:1147–1155
Ebina K, Hashimoto J, Kashii M et al (2017) The effects of switching daily teriparatide to oral bisphosphonates or denosumab in patients with primary osteoporosis. J Bone Miner Metab 35:91–98
Niimi R, Kono T, Nishihara A, Hasegawa M, Kono T, Sudo A (2018) Efficacy of switching from teriparatide to bisphosphonate or denosumab: a prospective, randomized, open-label trial. JBMR Plus 2:289–294
Park CH, Yoo JI, Choi CH, Suh YS (2020) The impact of sequential therapy from short-term teriparatide to denosumab compared with denosumab alone in patients with osteoporotic hip fracture: a 1-year follow-up study. BMC Musculoskelet Disord 21:751
Langdahl B (2020) Treatment of postmenopausal osteoporosis with bone-forming and antiresorptive treatments: combined and sequential approaches. Bone 139:115516
Muschitz C, Kocijan R, Fahrleitner-Pammer A et al (2014) Overlapping and continued alendronate or raloxifene administration in patients on teriparatide: effects on areal and volumetric bone mineral density—the CONFORS Study. J Bone Miner Res 29:1777–1785
Japan Osteoporosis Society (2015) Japanese 2015 guidelines for prevention and treatment of osteoporosis (in Japanese). http://www.josteo.com/ja/index.html. Accessed 14 December 2021
Rizzoli R, Reginster JY, Boonen S et al (2011) Adverse reactions and drug-drug interactions in the management of women with postmenopausal osteoporosis. Calcif Tissue Int 89:91–104
Adler RA, El-Hajj Fuleihan G, Bauer DC et al (2016) Managing osteoporosis in patients on long-term bisphosphonate treatment: report of a Task Force of the American Society for Bone and Mineral Research. J Bone Miner Res 31:16–35
Acknowledgements
The authors would like to thank those who participated as clinical investigators in JOINT-05. The authors would also like to express their thanks to the chairman, Dr. Itsuo Gorai and the members of the central ethics committee for the JOINT trials. The authors would also like to thank FORTE Science Communications (www.forte-science.co.jp) for professional assistance in preparing this manuscript.
Funding
This study was funded by the Public Health Research Foundation and Asahi Kasei Pharma Corp.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All the procedures performed in this study were in accordance with the Clinical Trials Act of the Japanese Ministry of Health, Labor and Welfare and with the 1964 Helsinki declaration and its later amendments. The protocol was approved by the certified review board of Toranomon Hospital and the central ethics committee of the Adequate Treatment of Osteoporosis research group.
Informed consent
Informed consent was obtained from all the individual participants included in the study.
Competing interests
H. Hagino has received lecture fees or grants outside the submitted work from Amgen Inc., Asahi Kasei Pharma Corp., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Eli Lilly Japan Co., Ltd., Mitsubishi Tanabe Pharma Corp., Mochida Pharma Corp., Ono Pharmaceutical Co., Ltd., Pfizer Inc., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Co., Ltd., and UCB Japan.
T. Sugimoto has received research grants from Asahi Kasei Pharma Corp., Astellas Pharma, Taisho Toyama Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Pfizer, and Teijin Pharma Ltd., as well as consulting fees from Kissei Pharmaceutical Co., Ltd., Shimadzu Corp., and Takeda Pharmaceutical Co., Ltd.
S. Tanaka has received lecture fees from Bayer Yakuhin, Amgen Astellas BioPharma K.K., and Research Institute of Healthcare Data Science. He has received consultation fees and outsourcing fees from Daiichi Sankyo Company, Limited, Boehringer Ingelheim, Satt, and the Public Health Research Foundation. He has received research grants from the Japan Agency for Medical Research and Development, the Japanese Ministry of Health Labor and Welfare, the Japanese Ministry of Education, Science, and Technology, and Novo Nordisk. He engaged in a research project of the Japan Agency for Medical Research and Development.
T. Sone has received research grants from Astellas Pharma, Eisai, Daiichi-Sankyo, Chugai Pharmaceutical, and Eli Lilly Japan, as well as consulting and/or lecture fees from Asahi Kasei Pharma, MSD, and Daiichi-Sankyo.
T. Nakamura has received personal fees and other from Asahi Pharma, Teijin Pharma, Daiichi-Sankyo Pharma, UCB Pharma, Amgen Inc., Astellas Pharma, and Chugai Pharma, as well as personal fees and others from MERCK.
S. Soen has received consulting fees, speaking fees, and/or honoraria from Asahi Kasei Pharma, Amgen, Astellas Pharma, Daiichi Sankyo, Eli Lilly Japan, Teijin Pharma, and UCB Japan.
S. Mori, Y. Mitomo, and K. Takahashi have nothing to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mori, S., Hagino, H., Sugimoto, T. et al. Sequential therapy with once-weekly teriparatide injection followed by alendronate versus monotherapy with alendronate alone in patients at high risk of osteoporotic fracture: final results of the Japanese Osteoporosis Intervention Trial-05. Osteoporos Int 34, 189–199 (2023). https://doi.org/10.1007/s00198-022-06570-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06570-0