Abstract
Summary
In this post hoc analysis, we assessed romosozumab efficacy and safety in European patients enrolled in FRAME. Romosozumab treatment through 12 months, followed by denosumab for a further 24 months, resulted in early and sustained risk reduction for major fracture categories, associated with large gains in bone mineral density.
Introduction
In the multinational FRAME phase 3 trial of romosozumab in postmenopausal women with osteoporosis, marked differences between clinical and non-vertebral fracture outcomes were observed among patients from Central and Southern America versus rest of world. This post hoc analysis assessed romosozumab efficacy and safety in European patients enrolled in the FRAME trial and extension study.
Methods
In FRAME (NCT01575834), patients were randomised 1:1 to romosozumab 210 mg or placebo monthly (QM) for 12 months, followed by open-label denosumab 60 mg Q6M to month 36, including a 12-month extension study. We report incidence of major fracture outcomes, bone mineral density (BMD) change from baseline and safety for European patients enrolled in FRAME.
Results
In FRAME, 3013/7180 (41.96%) patients were European; 1494 received romosozumab and 1519 received placebo. Through 12 months, romosozumab reduced fracture risk versus placebo for non-vertebral fracture (1.4% versus 3.0%; p = 0.004), clinical fracture (1.4% versus 3.6%; p < 0.001), new vertebral fracture (0.4% versus 2.1%; p < 0.001) and major osteoporotic fracture (0.9% versus 2.8%; p < 0.001), with results sustained through 36 months following transition to denosumab. Hip fractures were numerically reduced with romosozumab at month 12 (0.2% versus 0.6%; p = 0.092). Romosozumab increased BMD versus placebo at month 12; all patients in the romosozumab and placebo groups experienced further increases by month 36 after transition to denosumab. Adverse events were balanced between groups.
Conclusions
Among European patients in FRAME, romosozumab resulted in early and sustained risk reduction for all major fracture categories, associated with large BMD gains that continued after transition to denosumab.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with osteoporosis are at an increased risk of fracture, morbidity and mortality [1, 2]. Gains in bone mineral density (BMD) are associated with fracture risk reductions across all fracture categories [3]; therefore, treatments that can rapidly increase BMD, such as romosozumab [4], should be expected to reduce fracture risk across the whole spectrum of osteoporotic fractures [5].
In the phase 3 Fracture Study in Postmenopausal Women with Osteoporosis (FRAME; NCT01575834), romosozumab treatment through 12 months led to substantial gains in BMD as compared with placebo, associated with rapid reductions in the risk of vertebral and clinical fractures [6, 7]. Reductions in fracture risk and gains in BMD observed with romosozumab were sustained after transition to open-label anti-resorptive denosumab treatment through to the end of the primary analysis period at month 24 and through to month 36 after a 12-month extension phase [6, 8]. However, differences in non-vertebral fracture risk were noted among trial populations from different geographical regions and the reduction in the risk of non-vertebral fracture did not achieve statistical significance for all patients enrolled in FRAME through 12 months [6, 9].
Here we report a post hoc analysis to assess the efficacy and safety of romosozumab among European women with postmenopausal osteoporosis enrolled in the FRAME phase 3 trial and extension study. These analyses provide details on the European cohort of the FRAME study not previously reported. In the context of increased accessibility of romosozumab in several European countries, this information may help to better inform treatment decisions for women with postmenopausal osteoporosis.
Methods
Study design and patients
The post hoc analyses reported here are based on data from the FRAME phase 3, international, randomised, double-blinded, placebo-controlled, parallel-group trial [6, 8]. The study was approved by an independent ethics committee (IEC) or institutional review board (IRB) for each study centre. Informed consent was obtained from all individual participants included in the study. Patients included in these post hoc analyses were enrolled from the European Union (EU27) plus Switzerland and the UK, herein referred to as European patients.
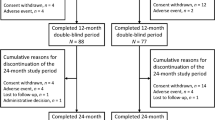
In FRAME, patients were randomised 1:1 to receive either 210 mg romosozumab subcutaneously or placebo monthly for 12 months. After 12 months, patients entered an open-label period and received 60 mg denosumab subcutaneously every 6 months for a further 24 months, including a 1-year extension study from months 24–36 (Fig. 1) [6, 8]. During the open-label period, blinding to the initial treatment allocation was maintained. Full inclusion and exclusion criteria have been described previously [6]. Briefly, all patients included in FRAME were ambulatory postmenopausal women aged 55–90 with a BMD T-score of − 2.5 to − 3.5 at the total hip or femoral neck. Exclusion criteria included a history of hip fracture, any severe or more than two moderate vertebral fractures, or exposure to treatments affecting bone metabolism (within washout periods as defined in the study protocol) [6].
Study procedures
Study procedures have previously been described in full [6]. Briefly, lateral radiographs of the spine were assessed for the presence of vertebral fractures at baseline and for the presence of new vertebral fractures (defined as those which occurred on-study) every 12 months thereafter, or, when a patient experienced back pain suggestive of vertebral fracture. Radiographs were assessed at a central imaging vendor (Bioclinica) and fracture severity was graded using the Genant semiquantitative grading system; assessors were blinded to treatment allocation [6]. Non-vertebral, clinical, hip and major osteoporotic fractures were assessed at the time that the event occurred and were adjudicated blinded to treatment allocation at the central imaging vendor (Bioclinica). Clinical fractures included clinical vertebral and non-vertebral fractures that were associated with signs and/or symptoms indicative of a fracture. Major osteoporotic fractures included clinical vertebral fractures and fractures of the hip, forearm or humerus that were not associated with high trauma severity or a pathologic fracture.
BMD measurements at the lumbar spine and proximal femur were performed by dual-energy X-ray absorptiometry (DXA) at baseline and every 12 months thereafter using Lunar or Hologic bone densitometers. DXA scans were processed and analysed blinded to treatment at the central imaging vendor (Bioclinica).
Statistical analysis
In these post hoc analyses, incidence of fracture is reported for new vertebral fracture, non-vertebral fracture, clinical fracture, hip fracture and major osteoporotic fracture. Incidence of new vertebral fracture was analysed by a logistic regression model adjusted for age and prevalent vertebral fracture stratification variables. Data are reported with odds ratios and 95% confidence intervals (CIs) for all patients included in the analysis set for new vertebral fractures. Missing data for new vertebral fractures were imputed by carrying forward the last non-missing post-baseline value prior to the missing value (LOCF). Incidences of all other fracture categories were analysed by a Cox proportional hazards model adjusted for age and prevalent vertebral fracture stratification variables. Data are reported with hazard ratios and 95% CIs for all patients included in the post hoc analyses.
Changes in BMD are reported as the least-squares mean percentage change from baseline. Least-squares mean percentage change from baseline was based on an ANCOVA model adjusted for age and prevalent vertebral fracture stratification variables, baseline value, machine type and baseline value-by-machine type interaction. Changes in BMD are reported with 95% CIs for patients who had a baseline and at least one post-baseline BMD measurement. Missing data were imputed by LOCF. Given the post hoc nature of these analyses, comparisons between treatment groups for the incidence of fracture and changes in BMD were not pre-specified. Therefore, all reported p values are nominal.
The incidences of adverse events (AEs) are reported for all included patients who received at least one dose of romosozumab or placebo during the double-blinded period of the trial. Data are reported for month 0–12 and month 0–36; data through 36 months are cumulative and include all AEs that occurred during the double-blinded period, the open-label period and the extension period of the trial. AEs are reported by preferred term, coded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 19.1.
Results
Patient disposition and baseline characteristics
Of the 7180 patients enrolled in the FRAME trial, 3013 (42%) patients were enrolled from EU27, plus Switzerland and the UK and were included in these analyses (Fig. 1). A total of 2597 (86.2%) European patients completed the 12-month double-blinded period of the trial and 2323 (77.1%) completed through to 36 months, including the 12-month extension study. The most common reasons for discontinuation through 36 months were consistent with results from the global study: withdrawal of consent (n = 393; 13.0%), adverse event (n = 70; 2.3%), other (n = 61; 2.0%) and death (n = 57; 1.9%).
Baseline demographics and characteristics for European patients enrolled in FRAME were balanced between treatment groups. Baseline characteristics in both treatment groups were similar to the overall FRAME population, with the exception of a higher FRAX score (for major osteoporotic fracture and hip fracture) and a greater prevalence of prior osteoporotic fracture (Table 1). The average age was 70.4 years and the mean ± standard deviation BMD T-score was − 2.52 ± 1.06 at the lumbar spine, − 2.43 ± 0.48 at the total hip and − 2.72 ± 0.28 at the femoral neck. A total of 1279 (42.4%) patients had experienced a prior osteoporotic fracture at or after the age of 45 years and 620 (20.6%) patients had at least one prevalent vertebral fracture. The mean 10-year probability of major osteoporotic fracture (based on FRAX® version 3.9, calculated with BMD) among European patients enrolled in FRAME was slightly higher than the mean probability for all patients enrolled in the global FRAME study (16.3% versus 13.4%, respectively). Similarly, the mean 10-year probability of hip fracture (FRAX® version 3.9, calculated with BMD) was higher among European patients enrolled in FRAME compared with the global FRAME study population (7.2% versus 5.9%, respectively).
Incidence of fracture
Through 12 months, romosozumab reduced the incidence of all fracture categories versus placebo (Figs. 2 and 3). At month 12, the incidence of new vertebral fractures was significantly lower among patients treated with romosozumab versus placebo (incidence 0.4% [6 of 1338 patients] in the romosozumab group versus 2.1% [29 of 1368 patients] in the placebo group; odds ratio, 0.21 [0.09, 0.52]; p < 0.001; Fig. 2). Furthermore, reductions in the incidence of new vertebral fractures were sustained through 24 months and 36 months after patients transitioned from romosozumab to denosumab compared with patients who transitioned from placebo to denosumab (Fig. 2).
Incidence of new vertebral fracture through 36 months. Incidences of new vertebral fracture are presented with odds ratios and 95% confidence intervals based on a logistic regression model adjusted for age and prevalent vertebral fracture stratification variables. Missing values were imputed by carrying forward the last non-missing post-baseline value prior to the missing value (LOCF). LOCF, last observation carried forward; n, number of patients in analyses for new vertebral fracture
Incidence of new fracture through 36 months. Incidences of (a) non-vertebral fracture, (b) clinical fracture, (c) hip fracture and (d) major osteoporotic fracture are presented with hazard ratio estimates and 95% confidence intervals based on a Cox proportional hazards model adjusted for age and prevalent vertebral fracture stratification variables. n, number of patients randomised
Similarly, the incidences of non-vertebral fracture, clinical fracture and major osteoporotic fracture were significantly reduced in romosozumab-treated versus placebo-treated patients. Through month 12, 1.4% (21 of 1494 patients) in the romosozumab group and 3.0% (45 of 1519 patients) in the placebo group experienced a non-vertebral fracture (hazard ratio, 0.47 [0.28, 0.79]; p = 0.004; Fig. 3a); 1.4% (21 of 1494 patients) in the romosozumab group and 3.6% (54 of 1519 patients) in the placebo group experienced clinical fractures (hazard ratio, 0.39 [0.24, 0.65]; p < 0.001 Fig. 3b), whilst a total of 0.9% (14 of 1494 patients) in the romosozumab group and 2.8% (42 of 1519 patients) in the placebo group experienced major osteoporotic fractures through month 12 (hazard ratio, 0.34 [0.19, 0.62]; p < 0.001; Fig. 3d). Incidences of hip fracture were numerically reduced with romosozumab (Fig. 3c). Reductions in the incidence of non-vertebral, clinical, major osteoporotic and hip fracture categories were sustained through 24 months and 36 months in patients who received romosozumab followed by denosumab compared with patients who received placebo followed by denosumab (Fig. 3).
Bone mineral density change from baseline
Romosozumab increased BMD at the lumbar spine, total hip and femoral neck by 12 months. At month 12, the least-squares mean percentage change from baseline in BMD was greater with romosozumab versus placebo by 12.3 percentage points (95% CI 11.9, 12.6; p < 0.001) at the lumbar spine (Fig. 4a), 5.2 percentage points (95% CI 5.0, 5.5; p < 0.001) at the total hip (Fig. 4b) and 5.0 percentage points (95% CI 4.7, 5.4; p < 0.001) at the femoral neck (Fig. 4c).
Bone mineral density percent change from baseline through 36 months. Least-squares mean percentage change in BMD from baseline at the (a) lumbar spine, (b) total hip and (c) femoral neck is reported with 95% confidence intervals. Data were based on an ANCOVA model adjusted for treatment, age and prevalent vertebral fracture stratification variables, baseline value, machine type and baseline value-by-machine type interaction; *p < 0.001. Missing data were imputed by carrying forward the last non-missing post-baseline value prior to the missing value (LOCF). BMD, bone mineral density; LOCF, last observation carried forward; n, number of patients with BMD values at baseline and at least one post-baseline visit
At month 24 and month 36, after all patients had transitioned to denosumab, patients from both the placebo and romosozumab groups experienced further gains in BMD. However, the least-squares mean percentage change from baseline in BMD at the lumbar spine, total hip and femoral neck remained significantly greater for patients who received romosozumab versus placebo prior to transitioning to denosumab (p < 0.001 for all comparisons; Fig. 4).
Safety
Overall, the incidence of AEs was balanced between treatment groups during the double-blinded period (months 0–12) and during the total study period, including the 12-month extension (months 0–36) (Table 2). Through 36 months, AEs were reported in 88.5% of patients treated with romosozumab followed by denosumab and 90.4% of patients treated with placebo followed by denosumab (Table 2). The incidences of serious AEs, AEs leading to discontinuation and treatment-related AEs were low and balanced between groups.
Through 12 months, during the double-blinded period of the trial, the most common AEs (reported in more than 10% of patients) were nasopharyngitis (reported by 15.3% of patients treated with romosozumab and 15.4% with placebo) and arthralgia (reported by 10.6% of patients treated with romosozumab and 10.0% with placebo) (Table 2).
Consistent with overall results previously reported for the FRAME trial [6], the incidence of serious adjudicated cardiovascular AEs was similar between groups through 12 months (reported by 1.4% of patients treated with romosozumab and 1.5% with placebo). The incidence remained low and similar between groups through 36 months; 4.0% of patients who transitioned from romosozumab to denosumab and 4.1% of patients who transitioned from placebo to denosumab reported serious adjudicated cardiovascular AEs (Table 2).
There was one incidence of adjudicated osteonecrosis of the jaw that occurred in the romosozumab treatment group during the first year of the trial. The event was associated with poorly fitted dentures and was considered not related to treatment by the investigator. No events adjudicated as atypical femoral fracture occurred in either treatment group through 36 months (Table 2). The incidence of injection site reactions was higher with romosozumab versus placebo through 12 months (5.5% versus 2.3%; Table 2). None of the injection site reactions was reported as serious. Incidences of other AEs of interest including malignancy, osteoarthritis and hypersensitivity were balanced between the two treatment groups through 36 months (Table 2).
Discussion
In these post hoc analyses reporting data for European patients enrolled in the FRAME phase 3 trial, romosozumab treatment led to significant reductions in the risk of new vertebral, clinical, non-vertebral and major osteoporotic fractures, in addition to numerically fewer hip fractures.
Previous studies have identified relationships between ethnicity, fracture risk and anti-fracture efficacy [9,10,11,12]. In the global FRAME study population, the reduction in non-vertebral fracture risk with romosozumab did not achieve statistical significance [6]. Further subgroup analyses confirmed that the incidence of non-vertebral fractures was low in patients enrolled from Central and Southern America compared with the rest of the world, consistent with the lower FRAX® score in Central and Southern American patients enrolled in the trial [6, 9]. In comparison, the European patients included in the analyses reported here had a higher fracture risk than the overall population enrolled in the global FRAME trial, as denoted by differences in baseline FRAX® score and the higher incidence of fracture in the placebo-treated patients. In support of these findings, previous post hoc analyses from the FRAME trial reported that the lower anti-fracture efficacy of romosozumab in Central and Southern American patients was not due to ineffectiveness of romosozumab in these patients, but was in fact impacted by the low non-vertebral fracture risk in this population [9]. Furthermore, significant interactions between romosozumab anti-fracture efficacy and baseline FRAX® have been reported, in which greater efficacy of romosozumab has been observed for patients with high baseline fracture risk [13].
Variations in fracture risk between geographies have been acknowledged previously [12], with such variations due to both genetic and environmental factors [11, 14]. Previous results from FRAME reported that patients from Central and Southern America had a lower baseline height than patients from other regions, and that fracture risk was lower despite a consistent number of falls, suggesting that skeletal differences may have contributed to a lower fracture risk in this population when considering the physical mechanics of a fall [9]. Aside from genetic and physical differences, other studies have reported on the relationship between dietary habits and osteoporosis [14], whilst a recent review summarising updates to the FRAX® tool note a number of new clinical risk factors that will increase applicability of FRAX®, including severity and treatment of type 2 diabetes, site, number of, and time since previous fracture, and prevalent falls, together emphasising the multi-factorial nature of fracture risk [15]. Further study would be required in order to determine whether such factors influenced the results observed in these post hoc analyses.
The increasing recognition of differences in fracture risk is also reflected in the development of country-specific FRAX® models [16,17,18,19,20]. Overall, the importance of ethnicity in determining fracture risk, alongside other factors such as time since fracture [21], is increasingly being recognised as a consideration for the personalised approach to osteoporosis treatment [10].
In these post hoc analyses, European patients enrolled in FRAME benefitted from significant reductions in the incidences of non-vertebral fracture and major osteoporotic fracture, observed through 12 months with romosozumab versus placebo. Importantly, the treatment effect of romosozumab was sustained after both treatment groups transitioned to anti-resorptive denosumab therapy; the incidence of all fracture categories was reduced for patients who received romosozumab followed by denosumab compared with patients who received placebo followed by denosumab. Since fracture risk reduction is influenced by baseline fracture risk, patients at medium, high or very high risk of fracture may experience greater benefits from treatment compared with those at low risk of fracture. Supportive of these findings, recent post hoc analyses from the first year of the FRAME phase 3 trial demonstrated that the efficacy of romosozumab for the reduction of clinical fracture, osteoporotic fracture and major osteoporotic fracture was significantly greater compared with placebo in patients at high baseline fracture risk [13]. Similar results were reported in the FREEDOM trial of denosumab in osteoporosis, in which patients at high risk of fracture experienced greater reductions in risk of clinical fracture and on-study fractures, likely due to their higher baseline fracture risk [22, 23].
Previous studies have reported increases in bone formation markers and reductions in bone resorption markers following treatment with romosozumab, which translate into rapid and substantial gains in BMD [6, 24, 25]. Changes in BMD observed in these post hoc analyses for patients enrolled from Europe were also noteworthy and consistent with the rapid and large gains previously reported [6, 8]. Through 12 months, greater gains in BMD were observed at the lumbar spine, total hip and femoral neck with romosozumab versus placebo and increases in BMD further improved through 36 months after transition from romosozumab to denosumab.
Overall, among European patients enrolled in FRAME, the incidence of AEs was balanced between treatment groups through 12 and 36 months. The most common adverse events during the 12-month double-blinded treatment period were nasopharyngitis and arthralgia; the incidence of these events was similar between romosozumab and placebo treatment groups. The incidence of serious cardiovascular events and cardiovascular events leading to death were low and similar between treatment groups through 12 and 36 months.
The post hoc analysis reported here includes a number of strengths; the size of the groups in these analyses were large and the data are reported from a controlled clinical trial with quality adjudicated fracture and BMD outcomes. Additionally, the countries included in these sub-analyses include different ethnicities and therefore a range of baseline risks. Limitations of this analysis include the fact that the data were collected during a clinical trial with defined patient inclusion criteria and therefore may not fully reflect that of a real-world population. Furthermore, given the post hoc nature of this study and that these sub-analyses were not pre-specified, the power to detect differences in efficacy and safety of romosozumab in these analyses may be limited. Despite this, significant reductions in the risk of new-vertebral, non-vertebral, clinical and major osteoporotic fractures were observed. In future, real-world data reporting on treatment with romosozumab and the romosozumab-denosumab sequence may provide further important insights for the management of osteoporosis in patients at high risk of fracture, with or without prior fracture.
In conclusion, among European patients enrolled in the FRAME phase 3 trial and extension, treatment with romosozumab through 12 months followed by denosumab for a further 24 months resulted in early and sustained reductions in risk of major fracture categories, associated with substantial gains in BMD.
Data availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinical-data-transparency-practices/clinical-trial-data-sharing-request/.
References
Nazrun AS, Tzar MN, Mokhtar SA, Mohamed IN (2014) A systematic review of the outcomes of osteoporotic fracture patients after hospital discharge: morbidity, subsequent fractures, and mortality. Ther Clin Risk Manag 10:937–948. https://doi.org/10.2147/TCRM.S72456
Laurs-van Geel TACM, Center JR, Geusens PP, Dinant G-J, Eisman JA (2010) Clinical fractures cluster in time after initial fracture. Maturitas 67:339–342. https://doi.org/10.1016/j.maturitas.2010.09.002
Bouxsein ML, Eastell R, Lui L-Y et al (2019) Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res 34:632–642. https://doi.org/10.1002/jbmr.3641
Padhi D, Jang G, Stouch B, Fang L, Posvar E (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26:19–26. https://doi.org/10.1002/jbmr.173
Black DM, Bauer DC, Vittinghoff E et al (2020) Treatment-related changes in bone mineral density as a surrogate biomarker for fracture risk reduction: meta-regression analyses of individual patient data from multiple randomised controlled trials. Lancet Diabetes Endocrinol 8:672–682. https://doi.org/10.1016/S2213-8587(20)30159-5
Cosman F, Crittenden DB, Adachi JD et al (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375:1532–1543. https://doi.org/10.1056/NEJMoa1607948
Cosman F, Crittenden DB, Ferrari S, Khan A, Lane NE, Lippuner K, Matsumoto T, Milmont CE, Libanati C, Grauer A (2018) FRAME study: the foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J Bone Miner Res 33:1219–1226. https://doi.org/10.1002/jbmr.3427
Lewiecki EM, Dinavahi RV, Lazaretti-Castro M, Ebeling PR, Adachi JD, Miyauchi A, Gielen E, Milmont CE, Libanati C, Grauer A (2019) One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: results of the FRAME extension study. J Bone Miner Res 34:419–428. https://doi.org/10.1002/jbmr.3622
Cosman F, Crittenden DB, Ferrari S, Lewiecki EM, Jaller-Raad J, Zerbini C, Milmont CE, Meisner PD, Libanati C, Grauer A (2018) Romosozumab FRAME study: a post hoc analysis of the role of regional background fracture risk on nonvertebral fracture outcome. J Bone Miner Res 33:1407–1416. https://doi.org/10.1002/jbmr.3439
Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM (2005) Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res 20:185–194. https://doi.org/10.1359/jbmr.041007
Kanis JA, Odén A, McCloskey EV, Johansson H, Wahl DA, Cooper C (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 23:2239–2256. https://doi.org/10.1007/s00198-012-1964-3
Cauley JA (2011) Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res 469:1891–1899. https://doi.org/10.1007/s11999-011-1863-5
McCloskey EV, Johansson H, Harvey NC, Lorentzon M, Shi Y, Kanis JA (2021) Romosozumab efficacy on fracture outcomes is greater in patients at high baseline fracture risk: a post hoc analysis of the first year of the frame study. Osteoporos Int 32:1601–1608. https://doi.org/10.1007/s00198-020-05815-0
Xu J, Li S, Zeng Y, Si H, Wu Y, Zhang S, Shen B (2022) Assessing the association between important dietary habits and osteoporosis: a genetic correlation and two-sample mendelian randomization study. Nutrients 14:2656. https://doi.org/10.3390/nu14132656
Vandenput L, Johansson H, McCloskey EV et al (2022) Update of the fracture risk prediction tool FRAX: a systematic review of potential cohorts and analysis plan. Osteoporos Int. https://doi.org/10.1007/s00198-022-06435-6
Lopez Gavilanez E, Johansson H, McCloskey E, Harvey NC, Segale Bajana A, Marriott Blum D, Navarro Grijalva M, Diaz Curiel M, Kanis JA (2019) Assessing the risk of osteoporotic fractures: the Ecuadorian FRAX model. Arch Osteoporos 14:93. https://doi.org/10.1007/s11657-019-0644-8
Lalmohamed A, Welsing PMJ, Lems WF, Jacobs JWG, Kanis JA, Johansson H, De Boer A, De Vries F (2012) Calibration of FRAX ® 3.1 to the Dutch population with data on the epidemiology of hip fractures. Osteoporos Int 23:861–869. https://doi.org/10.1007/s00198-011-1852-2
Jaller-Raad JJ, Jaller-Char JJ, Lechuga-Ortiz JA, Navarro-Lechuga E, Johansson H, Kanis JA (2013) Incidence of hip fracture in Barranquilla, Colombia, and the development of a Colombian FRAX model. Calcif Tissue Int 93:15–22. https://doi.org/10.1007/s00223-013-9717-7
Zerbini CA, Szejnfeld VL, Abergaria BH, McCloskey EV, Johansson H, Kanis JA (2015) Incidence of hip fracture in Brazil and the development of a FRAX model. Arch Osteoporos 10:224. https://doi.org/10.1007/s11657-015-0224-5
Kebaetse M, Nkhwa S, Mogodi M et al (2021) A country-specific FRAX model for Botswana. Arch Osteoporos 16:90. https://doi.org/10.1007/s11657-021-00965-y
Johansson H, Siggeirsdóttir K, Harvey NC, Odén A, Gudnason V, McCloskey E, Sigurdsson G, Kanis JA (2017) Imminent risk of fracture after fracture. Osteoporos Int 28:775–780. https://doi.org/10.1007/s00198-016-3868-0
Kendler DL, Chines A, Brandi ML, Papapoulos S, Lewiecki EM, Reginster JY, Muñoz Torres M, Wang A, Bone HG (2019) The risk of subsequent osteoporotic fractures is decreased in subjects experiencing fracture while on denosumab: results from the FREEDOM and FREEDOM Extension studies. Osteoporos Int 30:71–78. https://doi.org/10.1007/s00198-018-4687-2
McCloskey EV, Johansson H, Oden A, Austin M, Siris E, Wang A, Lewiecki EM, Lorenc R, Libanati C, Kanis JA (2012) Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res 27:1480–1486. https://doi.org/10.1002/jbmr.1606
Ishibashi H, Crittenden DB, Miyauchi A, Libanati C, Maddox J, Fan M, Chen L, Grauer A (2017) Romosozumab increases bone mineral density in postmenopausal Japanese women with osteoporosis: a phase 2 study. Bone 103:209–215. https://doi.org/10.1016/j.bone.2017.07.005
Langdahl BL, Libanati C, Crittenden DB et al (2017) Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet 390:1585–1594. https://doi.org/10.1016/s0140-6736(17)31613-6
Acknowledgements
The authors thank the patients, the investigators and their teams who took part in this study. The authors also acknowledge Helen Chambers, DPhil, Costello Medical, UK, for publication coordination and Claire Hews, PhD, Costello Medical, UK, for medical writing and editorial assistance based on the authors’ input and direction.
Funding
This study was sponsored by UCB Pharma and Amgen Inc. This article was based on the original FRAME study (NCT01575834) sponsored by UCB Pharma and Amgen Inc. Support for third-party writing assistance for this article, provided by Claire Hews, PhD, Costello Medical, UK, was funded by UCB Pharma in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Author information
Authors and Affiliations
Contributions
Substantial contributions to study conception and design: BL, LCH, SF, ZW, AFP, EG, PL, EC, EJG, JT, MO, CL; substantial contributions to analysis and interpretation of the data: BL, LCH, SF, ZW, AFP, EG, PL, EC, EJG, JT, MO, CL; drafting the article or revising it critically for important intellectual content: BL, LCH, SF, ZW, AFP, EG, PL, EC, EJG, JT, MO, CL; final approval of the version of the article to be published: BL, LCH, SF, ZW, AFP, EG, PL, EC, EJG, JT, MO, CL.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Consent for publication
All the results presented in this article are in aggregate form, and no personally identifiable information was used for this study.
Conflict of interest
BL: received fees and honoraria for lectures and advice from Amgen, Eli Lilly, Gedeon-Richter, Gilead and UCB Pharma; research grants for Aarhus University Hospital from Amgen and Novo Nordisk. LCH: received advisory board honoraria from Alexion, Amgen, Kyowa Kirin International, Shire and UCB Pharma. SF: support from Agnovos, Alexion, Amgen and UCB Pharma; consultant for Agnovos, Alexion, Amgen, Boehringer, Labatec, Myovant, Radius and UCB Pharma. ZW: employee of Amgen and stockholder of Amgen. AFP: research grants (to institution): Amgen, Roche; consultancy/lecture/advisory board fees: Alexion, Amgen, Eli Lilly, Fresenius, Gedeon-Richter, Genericon, Ipsen, Ratiopharm, Roche, Sandoz, Sanofi-Aventis, Shire-Takeda, Stada and UCB Pharma. EG: consultancy fees, lecture fees and/or travel fees from Alexion, Amgen, Sandoz, Takeda and UCB Pharma. PL: grants and/or advisor from Amgen, UCB Pharma, Richter and Teva. EC: grants from Amgen. EJG: consultancy and/or speaker and/or Investigator for Amgen, Asofarma, Astellas, AstraZeneca, Boehringer, BMS, FAES, Helios-Fresenius, Italfarmaco, Janssen, Lilly, MSD, Mundipharma, Novo Nordisk, Sanofi, Shire, Technopharma, UCB Pharma and Viatris. JT: employee of UCB Pharma and stockholder of UCB Pharma. MO: employee of Amgen. CL: employee of UCB Pharma and stockholder of UCB Pharma.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 101944 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Langdahl, B., Hofbauer, L.C., Ferrari, S. et al. Romosozumab efficacy and safety in European patients enrolled in the FRAME trial. Osteoporos Int 33, 2527–2536 (2022). https://doi.org/10.1007/s00198-022-06544-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06544-2