Abstract
Summary
Anti-resorptive osteoporosis treatment might be more effective in patients with high bone turnover. In this registry study including clinical data, high pre-treatment bone turnover measured with biochemical markers was correlated with higher bone mineral density increases. Bone turnover markers may be useful tools to identify patients benefitting most from anti-resorptive treatment.
Introduction
In randomized, controlled trials of bisphosphonates, high pre-treatment levels of bone turnover markers (BTM) were associated with a larger increase in bone mineral density (BMD). The purpose of this study was to examine this correlation in a real-world setting.
Methods
In this registry-based cohort study of osteoporosis patients (n = 158) receiving antiresorptive therapy, the association between pre-treatment levels of plasma C-telopeptide of type I Collagen (CTX) and/or N-terminal propeptide of type I procollagen (PINP) and change in bone mineral density (BMD) at lumbar spine, total hip, and femoral neck upon treatment was examined. Patients were grouped according to their pre-treatment BTM levels, defined as values above and below the geometric mean for premenopausal women.
Results
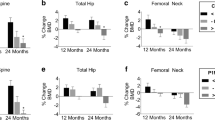
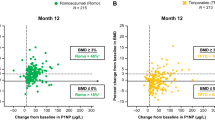
Pre-treatment CTX correlated with annual increase in total hip BMD, where patients with CTX above the geometric mean experienced a larger annual increase in BMD (p = 0.008) than patients with CTX below the geometric mean. The numerical pre-treatment level of CTX showed a similar correlation at all three skeletal sites (total hip (p = 0.03), femoral neck (p = 0.04), and lumbar spine (p = 0.0003)). A similar association was found for PINP where pre-treatment levels of PINP above the geometric mean correlated with a larger annual increase in BMD for total hip (p = 0.02) and lumbar spine (p = 0.006).
Conclusion
Measurement of pre-treatment BTM levels predicts osteoporosis patients’ response to antiresorptive treatment. Patients with high pre-treatment levels of CTX and/or PINP benefit more from antiresorptive treatment with larger increases in BMD than patients with lower pre-treatment levels.


Similar content being viewed by others
References
Cooper C, Campion G, Melton LJ 3rd (1992) Hip fractures in the elderly: a world-wide projection. Osteoporos Int 2(6):285–289
Vestergaard P, Rejnmark L, Mosekilde L (2005) Osteoporosis is markedly underdiagnosed: a nationwide study from Denmark. Osteoporos Int 16(2):134–141
Parfitt AM (1984) The cellular basis of bone remodeling: the quantum concept reexamined in light of recent advances in the cell biology of bone. Calcif Tissue Int 36(Suppl 1):S37-45
Jorgensen NR et al (2017) Comparison of two automated assays of BTM (CTX and P1NP) and reference intervals in a Danish population. Osteoporos Int 28(7):2103–2113
Michelsen J et al (2013) Reference intervals for serum concentrations of three bone turnover markers for men and women. Bone 57(2):399–404
Pols HA et al (1999) Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int 9(5):461–8
Cummings SR et al (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280(24):2077–2082
Jacques RM et al (2012) Relationship of changes in total hip bone mineral density to vertebral and nonvertebral fracture risk in women with postmenopausal osteoporosis treated with once-yearly zoledronic acid 5 mg: the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 27(8):1627–1634
Russell RG et al (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19(6):733–759
Allen MR et al (2010) Morphological assessment of basic multicellular unit resorption parameters in dogs shows additional mechanisms of bisphosphonate effects on bone. Calcif Tissue Int 86(1):67–71
Ramchand SK, Seeman E (2020) Reduced bone modeling and unbalanced bone remodeling: targets for antiresorptive and anabolic therapy. Handb Exp Pharmacol 262:423–450
Bouxsein ML et al (2019) Change in bone density and reduction in fracture risk: a meta-regression of published trials. J Bone Miner Res 34(4):632–642
Bauer DC et al (2006) Pretreatment levels of bone turnover and the antifracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res 21(2):292–299
Reyes C et al (2016) Real-life and RCT participants: alendronate users versus FITs’ trial eligibility criterion. Calcif Tissue Int 99(3):243–249
Vasikaran S et al (2011) International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med 49(8):1271–1274
Schmidt M, Pedersen L, Sorensen HT (2014) The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 29(8):541–549
Schmidt M et al (2015) The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 7:449–490
Pottegard A et al (2017) Data Resource profile: the Danish National Prescription Registry. Int J Epidemiol 46(3):798–798f
Bauer DC et al (2004) Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res 19(8):1250–1258
Eastell R et al (2011) Bone turnover markers and bone mineral density response with risedronate therapy: relationship with fracture risk and patient adherence. J Bone Miner Res 26(7):1662–1669
Saag KG et al (1998) Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med 339(5):292–9
Wasnich RD, Miller PD (2000) Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab 85(1):231–236
Cummings SR et al (2002) Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 112(4):281–289
Christgau S et al (2000) Serum CrossLaps for monitoring the response in individuals undergoing antiresorptive therapy. Bone 26(5):505–511
Clowes JA et al (2002) Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone 30(6):886–890
Schmidt M et al (2019) The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 11:563–591
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Niklas Rye Jørgensen declares that he has previously received assays as a donation from IDS Plc and Roche for research use but has no further conflict of interest. PE serves on the advisory board of UCB and Gedeon-Richter. BA reports speakers’ fees or consulting fees from Amgen, UCB, Kyowa-Kirin, Pharmacosmos, and Gedeon Richter and institutional research grants from UCB, Kyowa-Kiring, and Gedeon Richter. JEBJ reports advisory board membership in Eli Lilly, Amgen, Gedeon Richter, and UCB; received funding from Eli Lilly, Amgen, Alexion, and Samsung; and speaker’s fees from Eli Lilly, UCB, Amgen, Gedeon Richter, and Osaka. SEB, MSR, BH, SA, CDS, PH, PSO, LTJ, SM, KHR, and MFH have no conflicts of interest relevant to this study to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bønløkke, S.E., Rand, M.S., Haddock, B. et al. Baseline bone turnover marker levels can predict change in bone mineral density during antiresorptive treatment in osteoporotic patients: the Copenhagen bone turnover marker study. Osteoporos Int 33, 2155–2164 (2022). https://doi.org/10.1007/s00198-022-06457-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06457-0