Abstract
Summary
Duchenne muscular dystrophy is a progressive disease usually associated with loss of ambulation and progressive scoliosis. Immobilisation and glucocorticoid treatment are predisposing factors for reduced bone mineral density (BMD). Analysis of quantitative computed tomography revealed low BMD in thoracic and lumbar vertebrae in comparison to age- and sex-matched healthy controls.
Introduction
Evaluation of vertebral bone mineral density (BMD) in Duchenne Muscular Dystrophy (DMD) adolescents with untreated advanced scoliosis and comparison with the BMD values of healthy age-matched controls, based on quantitative computer tomography.
Methods
Thirty-seven DMD adolescents (age 15.6 ± 2.5 years) with spinal deformity were evaluated clinically and radiologically prior to definite spinal fusion and compared to 31 male and age-matched healthy individuals (age 15.7 ± 2.3 years). Data related to previous medical treatment, physiotherapy and ambulatory status was also analysed. Scoliotic curves were measured on plain sitting radiographs of the spine. The BMD Z-scores of the thoracic and lumbar vertebrae were calculated with QCTpro® (Mindways Software Inc., USA), based on data sets of preoperative, phantom pre-calibrated spinal computed tomography scans.
Results
A statistically significant lower BMD could be found in DMD adolescents, when compared to healthy controls, showing an average value for the lumbar spine of 80.5 ± 30.5 mg/cm3. Z-scores deteriorated from the upper thoracic towards the lower lumbar vertebrae. All but the uppermost thoracic vertebrae had reduced BMD values, with the thoracolumbar and lumbar region demonstrating the lowest BMD. No significant correlation was observed between BMD and the severity of the scoliotic curve, previous glucocorticoid treatment, cardiovascular impairment, vitamin D supplementation, non-invasive ventilation or physiotherapy.
Conclusion
DMD adolescents with scoliosis have strongly reduced BMD Z-scores, especially in the lumbar spine in comparison to healthy controls. These findings support the implementation of a standardised screening and treatment protocol.
Level of evidence/clinical relevance: therapeutic level III
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reduced bone mineral density (BMD) in osteopenia and loss of the trabecular bone structure via defective remodelling in osteoporosis lead to increased fracture risk. Whilst these entities are common amongst the elderly, the extent of their presence as secondary forms amongst adolescent patients suffering from degenerative muscle disorders, such as Duchenne Muscular Dystrophy (DMD), has only recently been examined [1,2,3]. Risk factors for reduced BMD by DMD patients include the loss of ambulation, as well as adverse effects of glucocorticoid (GC) therapy on bone metabolism [4] which can lead to vertebral fractures [5], even by non-ambulatory patients. Furthermore, up to 85% of DMD adolescents develop a progressive neuromuscular scoliosis (Fig. 1), resulting in a definite spinal fusion in the majority of cases [6]. Thus, reduced BMD may pose a problem for surgical treatment of DMD patients with scoliosis and also increase the subsequent complication risk by negatively influencing the correction potential, screw placement and anchorage as well as pain management, as reported for adult patients [7, 8].
To our knowledge, this cross-sectional cohort study is the first of its kind to evaluate the vertebral BMD of DMD adolescents with scoliosis in comparison to age- and sex-matched healthy controls, based on precalibrated, quantitative computer tomography (QCT) of the thoracic and lumbar vertebrae.
Material and methods
After approval by the institutional ethical review committee, we performed a cross-sectional cohort study on adolescent patients diagnosed with DMD and progressive spinal deformity without prior surgical spine treatment. All participants were informed about the purpose of the study, and oral and written consent was obtained from a parent and/or legal guardian for the evaluation of clinical and demographic data.
Between 2017 and 2021, 45 DMD adolescents received a standardised work-up in preparation for definite spinal fusion. Using a questionnaire, demographic and clinical data were acquired. Scoliotic curves were measured on standardised sitting anteroposterior (ap) and lateral radiographs using Centricity Enterprise Web Version 3.0 (GE Healthcare Medical Systems, Chicago, USA, 2006).
Forty-five asynchronous, phantom pre-calibrated CT scans (Somatom Definition AS, Siemens, Erlangen, Germany) of the whole spine, with 0.6 mm slice thickness, were performed for preoperative evaluation. A total of eight patients had to be excluded due to technical inconsistency of the calibration, leaving 37 participants (age 15.6 ± 2.5 years) for the BMD extraction. None of the DMD patients had vertebral fractures.
The control group consisted of 31 age-matched male patients (age 15.7 ± 2.3 years), undergoing a native CT (n = 16) or a contrast enhanced CT (n = 15) with the contrast agent Imeron® 350 (Bracco Imaging Deutschland GmbH, Konstanz, Germany) with an iodine concentration of 350 mg/ml and a maximal slice thickness of 0.6 to 0.75 mm. All examined persons were otherwise healthy participants, who received a CT to rule out or confirm acute conditions such as high-energy trauma (n = 21), urinary tract exam (n = 7), spondylolisthesis (n = 1), pneumothorax (n = 1) and acute abdomen (n = 1). Any fractured vertebrae or pathological vertebral findings were excluded from the BMD measurement.
CT data were evaluated using the software QCTpro® version 6.1 (Mindways Software Inc., Austin, TX, USA). The evaluation consisted of the axial alignment of the vertebrae, definition of the range of interest (ROI) within the area for measurement and extraction of the BMD values.
Due to the scoliotic deformity observed in DMD patients, the alignment to the transverse, coronal and sagittal planes for each segment as well as the ROI definitions was performed manually (Fig. 2a). To measure interobserver accuracy, the evaluation was carried out independently by two physicians. Measurements with deviation of 5% or more from the mean were revised. From a total of 612 values per examiner, 169 (27.6%) had to be revised. Causes for the differing values were the inclusion of non-trabecular structures in the ROI, such as cortical bone and neurovascular structures, insufficient alignment of the anatomical planes or false definition of the level of the evaluated segments.
Software QCTpro® for evaluating BMD. A The red and yellow areas show the ROI in the three anatomical planes, which should exclude cortical bone and neurovascular structures. B BMD results of the lumbar spine for a DMD patient, with Z-scores being provided based on normal values of the UCSF database [9,10,11,12,13]
CT scans of the control group with physiological vertebral anatomy and orientation were evaluated by one physician, as the process was straightforward and easily automated. The values obtained through contrast enhanced CT scans (BMDMDCT) were converted using the equation BMDQCT = 0.96 × BMDMDCT − 20.9 mg/mL as described by Bauer et al. (2007) [9], to compensate for the higher BMD results due to the contrast agent. This equation was initially conceived for the lumbar vertebrae L1 until L3. For this reason, we also statistically compared the adjusted results from the contrast enhanced CT scans to those of the native ones, which ruled out a significant difference (p < 0.05).
The Z-score, which is an expression of the standard deviation (SD) observed within the BMD values of the patient group when compared to the mean BMD value of the age- and sex-matched control group, was calculated [10]. Height-adjusted Z-scores (HAZ) for growth compensation are not needed by QCT-based vBMD, since all three dimensions are already included, which is in contrast to dual-energy-x-ray-absorptiometry (DXA) [11]. Whilst the software automatically provides Z-scores based on reference BMD values acquired from the database of the University of California, San Francisco (UCSF), through studies between 1985 and 1989 [12,13,14,15,16] (Fig. 2B), we saw fit to use for demographic reasons local control data for Z-score calculations. Initially, however, we did use the Z-score results provided by the software to confirm the consistency of our own reference group. BMD values from both the DMD and control groups were compared for each thoracic and lumbar vertebra. Furthermore, Z-scores for each vertebra of the DMD patients were calculated and compared to the threshold Z-scores based on the critical BMD values of 120 mg/cm3 and 80 mg/cm3, respectively, as defined in the guidelines of the American College of Radiology (ACR) [17] that are also implemented in QCT evaluations [17]. The threshold Z-scores were calculated based on the average BMD for each vertebra in the control group (BMDctrl) and its standard deviation (SDctrl) with the following formulae: Z1 = (120-BMDctrl)/SDctrl and Z2 = (80-BMDctrl)/SDctrl. Statistical differences between BMD values within the DMD patient group were assessed according to the criteria outlined in Table 1.
Statistical analyses using post-hoc tests were carried out using the statistical software package GraphPad Prism® and Excel® (Microsoft Corporations, Redmond, USA). An unpaired t-test was used to compare the BMD and Z-Scores of each individual vertebra between the control group and the DMD group, as well as between the subgroups of the DMD patients, according to possibly influencing factors of the BMD. The subgroup criteria were as follows: a scoliotic curve below 40° (n = 9) versus at least 40° (n = 28); GC treatment (n = 26) versus GC-naive patients (n = 11); medication for beginning dilated cardiomyopathy, such as beta-receptor blockers (n = 10) versus no cardiovascular treatment (n = 27); vitamin D supplementation with 1000 international units per day (n = 32) versus no vitamin D supplementation (n = 5); non-invasive ventilation (NIV) in form of continuous positive airway pressure (n = 12) versus no NIV therapy (n = 25) and regular physiotherapy (n = 15) versus no physiotherapy (n = 22). A simple linear regression analysis was additionally performed to investigate a potential correlation of scoliosis angle and BMD for each individual vertebra. A multiple regression analysis for the abovementioned parameters was not performed, as their implementation varied strongly from patient to patient. Data are presented as mean ± SD. Statistical significance was defined as p < 0.05 (*), p < 0.01 (**) and as p < 0.001 (***). Nomenclature is based on the recommendations of the 2003 Position Development Conference (PDC) of the International Society for Clinical Densitometry (ISCD) [18]
Results
The average BMD was significantly reduced (p < 0.001) in the DMD group when compared to the control group for every thoracic and lumbar vertebra, with the values progressively deteriorating from T1 towards L5 (Fig. 3).
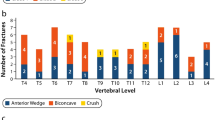
The initial consistency proofing of the control group based on the QCTpro® internal database showed relatively reduced, but normal, Z-scores (− 0.9 ± 1.0). This is in agreement with the World Health Organisation (WHO) recommendations for adults [19], as well as the ACR guidelines [17], which consider Z-scores above − 2.0 as normal. Thus, this allowed us to use these data as a reference for the calculation of the Z-scores in the DMD group (also known as gradient of risk [19]). The comparison of these results to the low bone mass thresholds as calculated with the above-described equations, with the exception of the upper thoracic vertebrae, reveals reduced bone mass for the remaining thoracic segments and further rapid deterioration of the bone mass for the thoracolumbar region and below. This gradient of risk is depicted in Fig. 4.
Average BMD Z-scores (blue) for DMD patients (n = 37) for each thoracic and lumbar vertebra. From the healthy age- and sex-matched healthy control group (n = 31) calculated Z-score thresholds for critical BMD values of 120 mg/cm3 (orange) and 80 mg/cm3 (red), respectively. A significant deterioration of BMD values is shown for the lower thoracic and lumbar region. T = thoracic; L = lumbar
The impact of demographic and clinical factors on BMD in DMD adolescents was assessed using the data provided by the patients in the form of a questionnaire (Table 1). No correlation was found between BMD values in DMD adolescents in respect to the severity of scoliosis (scoliosis angle < 40° versus > / = 40°), previous GC therapy, vitamin D3 supplementation (1000 international units (IU) per day) or cardiovascular impairment (with beta-receptor-blocker treatment for beginning dilated cardiomyopathy). In the same manner, NIV as an indicator for reduced lung function or supported standing therapy, reflecting the general health condition of the patients, had no statistical relevance as an influencing factor on BMD.
Discussion
This cohort study examines the vertebral BMD of the thoracic and lumbar spine in DMD adolescents with scoliosis based on quantitative computer tomography, and in doing so, highlights an approach that can provide more accurate measurements in comparison to DXA [11, 20]. The fine slice CT scans allow accurate volumetric BMD (vBMD) measurements and so overcomes inherent limitations of the areal, indirectly calculated vBMD obtained through DXA in cases of growing patients and severe spinal deformity [11, 20]. Our measurements demonstrate reduced BMD Z-scores for all thoracic and lumbar vertebral segments in DMD patients when compared to age- and sex-matched healthy controls, with the values of the lumbar region reflecting the highest risk for vertebral fractures due to low BMD.
Still, the diagnosis of osteoporosis in children and adolescents cannot be based on the same criteria as for adults and densitometry alone [17, 19]. According to the official paediatric positions of the International Society for Clinical Densitometry (ISCD), BMD Z-scores equal or below − 2.0 alone are not indicative of osteoporosis, nor do Z-scores above − 2.0 exclude a high fracture risk, especially in neuromuscular disorders. One or more vertebral compression fractures without adequate trauma could indicate osteoporosis. In the absence of such fractures, a clinically significant long bone fracture history, as defined by the ISCD, in combination with a BMD Z-score ≤ − 2.0 should lead to the diagnosis of osteoporosis [21]. In our patient group, because of external input from various hospitals, it was not possible to have access to other medical records in order to collect reliable information on non-vertebral fractures for statistical analysis. Secondary reduced BMD follows the gradual deterioration of muscle function and loss of ambulation in DMD adolescents [22], which occur at about 10 years of age [23].
At the same time, GC treatment reduces BMD and increases the risk of vertebral fractures due to low bone mineral mass [4]. According to a nationwide investigation of 832 DMD cases in the UK, the overall fracture incidence was more than four times higher in patients under long-term, daily therapy with deflazacort, in comparison to healthy boys [3]. Another retrospective study estimated vertebral fractures in up to one-third of the GC-treated DMD patients [24]. Slowly progressing, asymptomatic vertebral fractures account for almost 50% of these fractures with low BMD [25] and remain unnoticed in up to one-third of patients [26]. Moreover, these fractures can contribute in part to the loss of ambulation, since vertebral fractures may cause pain that is not always responsive to common analgesic drugs and may require specific medical treatment [27]. For this reason, international literature recommends routine screening for vertebral fractures in DMD adolescents with risk factors such as GC therapy or BMD Z-score deterioration alone, without previous fractures or symptoms like back pain [28].
On the positive side, GC treatment prolongs the ambulant phase of DMD patients [29] through its anti-inflammatory effect on skeletal muscle [30] and indirectly through growth impairment [31], with a shorter stature being biomechanically beneficial for retaining muscle function [32]. Since progression of scoliosis is closely related to loss of ambulation, long-term GC treatment of DMD patients halts the progression of spinal deformity through prolonged mobility [33]. Characteristically, scoliosis in DMD boys only exceeds a scoliosis angle of 30°, if the patient is wheelchair-bound [6]. Whilst 67 to 92% of steroid-naive DMD adolescents have progressive scoliosis, only up to 20% of the GC-treated patients show the same development [34, 35] and require surgical stabilisation [36]. Our results do not allow the distinction between whether or not continuous or intermittent GC treatment prevents scoliosis development in DMD adolescents, or merely delays its onset, since the patients are treated primarily in other institutions nationwide and therefore the therapy is not standardised. Thus, further and more detailed studies are required to clarify this issue.
Data delivered independently from researchers at the 170th European Neuromuscular Centre Workshop showed highly reduced lumbar BMD in non-ambulant DMD patients regardless of former GC treatment [37, 38]. Our study supports these results, since all 37 participants had loss of ambulation at the average age of 9.8 ± 2.3 years, and at least 5 years prior to the CT examinations from which BMD data were acquired. Along the same lines as this, although not directly comparable due to a higher dose and shorter duration, a study on patients requiring GC therapy for multiple sclerosis showed that although bone turn-over markers increased almost immediately after treatment initiation, this only persisted until 3 months after the end of therapy, and no difference was observed in BMD before or after 6 months of treatment [39]. This could also explain why we did not detect any difference in BMD between pretreated and GC-naïve DMD patients. Furthermore, the degree of scoliosis, vitamin D3 supplementation, cardiovascular impairment, NIV or supported standing therapy did not correlate to BMD data in DMD adolescents.
A limitation of this study is the authors’ specialisation on surgical scoliosis correction, which automatically leads to a specific, pre-sorted subgroup of DMD patients being treated, namely, those with advanced spinal deformity. As a result, these patients had ceased the GC treatment 4.0 ± 2.7 before the QCT evaluation, which may be long enough to influence any remaining catabolic effect this may have on the BMD measured. In addition, milder cases are not represented in our analysis and a follow-up relevant to this study is not possible after spinal fusion. Another inherent problem was that the demographic factors examined, such as GC medication, were highly variable as to dosage, duration and type of administration, depending on the originating clinic of the patient and the actual therapy regimes. The control group consisted of adolescents without spinal deformity. A scoliosis-matched control group would be able to rule out scoliosis itself as an influencing factor for reduced BMD.
Conclusions
The present study demonstrated with accurate volumetric BMD data that DMD patients with advanced scoliosis have significantly reduced BMD Z-scores compared to age- and sex-matched controls in the thoracic and lumbar vertebrae. All but the uppermost thoracic vertebra had reduced Z-scores, which rapidly deteriorated from the thoracolumbar region and downwards, thus reflecting the high risk for vertebral fractures due to low bone mineral mass. Whilst awareness on this subject is internationally increasing, there is still need for standardised and widely accepted screening and treatment protocols.
Availability of data and materials
Please contact author for data requests.
Abbreviations
- BMD:
-
Bone mineral density
- DMD:
-
Duchenne Muscular Dystrophy
- CT:
-
Computed tomography
- QCT:
-
Quantitative computer tomography
- ROI:
-
Range of interest
- HAZ:
-
Height-adjusted Z-scores
- DXA:
-
Dual-energy-x-ray-absorptiometry
References
Bell JM, Shields MD, Watters J et al (2017) Interventions to prevent and treat corticosteroid-induced osteoporosis and prevent osteoporotic fractures in Duchenne muscular dystrophy. Cochrane Database Syst Rev 1:CD010899. https://doi.org/10.1002/14651858.CD010899.pub2
Ward LM, Hadjiyannakis S, McMillan HJ et al (2018) Bone health and osteoporosis management of the patient with Duchenne Muscular Dystrophy. Pediatrics 142:S34–S42. https://doi.org/10.1542/peds.2018-0333E
Joseph S, Wang C, Bushby K et al (2019) Fractures and linear growth in a nationwide cohort of boys with Duchenne Muscular Dystrophy with and without glucocorticoid treatment: results from the UK NorthStar Database. JAMA Neurol 76:701. https://doi.org/10.1001/jamaneurol.2019.0242
Tian C, Wong BL, Hornung L et al (2016) Bone health measures in glucocorticoid-treated ambulatory boys with Duchenne muscular dystrophy. Neuromuscul Disord 26:760–767. https://doi.org/10.1016/j.nmd.2016.08.011
Singh A, Schaeffer EK, Reilly CW (2018) Vertebral fractures in Duchenne Muscular Dystrophy patients managed with deflazacort. J Pediatr Orthop 38:320–324. https://doi.org/10.1097/BPO.0000000000000817
Oda T, Shimizu N, Yonenobu K et al (1993) Longitudinal study of spinal deformity in Duchenne Muscular Dystrophy. J Pediatr Orthop 13:478–488. https://doi.org/10.1097/01241398-199307000-00012
Gupta A, Cha T, Schwab J et al (2021) Osteoporosis increases the likelihood of revision surgery following a long spinal fusion for adult spinal deformity. Spine J Off J North Am Spine Soc 21:134–140. https://doi.org/10.1016/j.spinee.2020.08.002
Yuan L, Zhang X, Zeng Y, et al (2021) Incidence, risk, and outcome of pedicle screw loosening in degenerative lumbar scoliosis patients undergoing long-segment fusion. Glob Spine J 21925682211017476. https://doi.org/10.1177/21925682211017477
Bauer JS, Henning TD, Müeller D et al (2007) Volumetric quantitative CT of the spine and hip derived from contrast-enhanced MDCT: conversion factors. Am J Roentgenol 188:1294–1301. https://doi.org/10.2214/AJR.06.1006
Carey JJ, Delaney MF (2010) T-scores and Z-scores. Clin Rev Bone Miner Metab 8:113–121. https://doi.org/10.1007/s12018-009-9064-4
Zemel BS, Leonard MB, Kelly A et al (2010) Height adjustment in assessing dual energy X-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab 95:1265–1273. https://doi.org/10.1210/jc.2009-2057
Cann CE, Genant HK, Kolb FO, Ettinger B (1985) Quantitative computed tomography for prediction of vertebral fracture risk. Bone 6:1–7. https://doi.org/10.1016/8756-3282(85)90399-0
Cann CE (1988) Quantitative CT for determination of bone mineral density: a review. Radiology 166:509–522. https://doi.org/10.1148/radiology.166.2.3275985
Block JE, Smith R, Glueer CC et al (1989) Models of spinal trabecular bone loss as determined by quantitative computed tomography. J Bone Miner Res Off J Am Soc Bone Miner Res 4:249–257. https://doi.org/10.1002/jbmr.5650040218
Gilsanz V, Varterasian M, Senac MO, Cann CE (1986) Quantitative spinal mineral analysis in children. Ann Radiol (Paris) 29:380–382
Gilsanz V, Gibbens DT, Roe TF et al (1988) Vertebral bone density in children: effect of puberty. Radiology 166:847–850. https://doi.org/10.1148/radiology.166.3.3340782
(2018) ACR–SPR–SSR practice guideline for the performance of quantitative computed tomography (QCT) bone, Available online: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/qct.pdf. Accessed 02 Feb 2022
Writing Group for the ISCD Position Development Conference (2004) Nomenclature and decimal places in bone densitometry. J Clin Densitom Off J Int Soc Clin Densitom 7:45–50. https://doi.org/10.1385/jcd:7:1:45
Weltgesundheitsorganisation (2003) Prevention and management of osteoporosis: report of a WHO scientific group; [WHO Scientific Group Meeting on Prevention and Management of Osteoporosis, Geneva, 7–10 April]. World Health Organization, Geneva
Ward RJ, Roberts CC, Bencardino JT et al (2017) ACR Appropriateness Criteria ® osteoporosis and bone mineral density. J Am Coll Radiol 14:S189–S202. https://doi.org/10.1016/j.jacr.2017.02.018
2019 ISCD Official Positions Pediatric; Available online: https://iscd.org/learn/official-positions/pediatric-positions/. Accessed 29 Mar 2022
Morgenroth VH, Hache LP, Clemens PR (2012) Insights into bone health in Duchenne Muscular Dystrophy. BoneKEy Rep 1: https://doi.org/10.1038/bonekey.2012.5
Archer JE, Gardner AC, Roper HP et al (2016) Duchenne Muscular Dystrophy: the management of scoliosis. J Spine Surg 2:185–194. https://doi.org/10.21037/jss.2016.08.05
King WM, Ruttencutter R, Nagaraja HN et al (2007) Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne Muscular Dystrophy. Neurology 68:1607–1613. https://doi.org/10.1212/01.wnl.0000260974.41514.83
Nakhla M, Scuccimarri R, Duffy KNW et al (2009) Prevalence of vertebral fractures in children with chronic rheumatic diseases at risk for osteopenia. J Pediatr 154:438–443. https://doi.org/10.1016/j.jpeds.2008.09.023
Mäkitie O, Doria AS, Henriques F et al (2005) Radiographic vertebral morphology: a diagnostic tool in pediatric osteoporosis. J Pediatr 146:395–401. https://doi.org/10.1016/j.jpeds.2004.10.052
Catalano A, Vita GL, Russo M et al (2016) Effects of teriparatide on bone mineral density and quality of life in Duchenne Muscular Dystrophy related osteoporosis: a case report. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA 27:3655–3659. https://doi.org/10.1007/s00198-016-3761-x
Birnkrant DJ, Bushby K, Bann CM et al (2018) Diagnosis and management of Duchenne Muscular Dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 17:347–361. https://doi.org/10.1016/S1474-4422(18)30025-5
Crabtree NJ, Adams JE, Padidela R et al (2018) Growth, bone health & ambulatory status of boys with DMD treated with daily vs. intermittent oral glucocorticoid regimen. Bone 116:181–186. https://doi.org/10.1016/j.bone.2018.07.019
Herbelet S, Rodenbach A, De Paepe B, De Bleecker JL (2020) Anti-inflammatory and general glucocorticoid physiology in skeletal muscles affected by Duchenne Muscular Dystrophy: exploration of steroid-sparing agents. Int J Mol Sci 21:4596. https://doi.org/10.3390/ijms21134596
Ward LM, Weber DR (2019) Growth, pubertal development, and skeletal health in boys with Duchenne Muscular Dystrophy. Curr Opin Endocrinol Diabetes Obes 26:39–48. https://doi.org/10.1097/MED.0000000000000456
Zatz M, Rapaport D, Vainzof M et al (1988) Relation between height and clinical course in Duchenne Muscular Dystrophy. Am J Med Genet 29:405–410. https://doi.org/10.1002/ajmg.1320290223
Ricotti V, Ridout DA, Scott E et al (2013) Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne Muscular Dystrophy. J Neurol Neurosurg Psychiatry 84:698–705. https://doi.org/10.1136/jnnp-2012-303902
Griggs RC, Miller JP, Greenberg CR et al (2016) Efficacy and safety of deflazacort vs prednisone and placebo for Duchenne Muscular Dystrophy. Neurology 87:2123–2131. https://doi.org/10.1212/WNL.0000000000003217
Alman BA, Raza SN, Biggar WD (2004) Steroid treatment and the development of scoliosis in males with Duchenne Muscular Dystrophy. J Bone Jt Surg 86:519–524. https://doi.org/10.2106/00004623-200403000-00009
Lebel DE, Corston JA, McAdam LC et al (2013) Glucocorticoid treatment for the prevention of scoliosis in children with Duchenne Muscular Dystrophy: long-term follow-up. J Bone Jt Surg-Am 95:1057–1061. https://doi.org/10.2106/JBJS.L.01577
Quinlivan R, Shaw N, Bushby K (2010) 170th ENMC international workshop: bone protection for corticosteroid treated Duchenne Muscular Dystrophy. 27–29 November 2009, Naarden. The Netherlands Neuromuscul Disord 20:761–769. https://doi.org/10.1016/j.nmd.2010.07.272
Catalano A, Vita GL, Bellone F et al (2022) Bone health in Duchenne Muscular Dystrophy: clinical and biochemical correlates. J Endocrinol Invest 45:517–525. https://doi.org/10.1007/s40618-021-01676-4
Dovio A, Perazzolo L, Osella G et al (2004) Immediate fall of bone formation and transient increase of bone resorption in the course of high-dose, short-term glucocorticoid therapy in young patients with multiple sclerosis. J Clin Endocrinol Metab 89:4923–4928. https://doi.org/10.1210/jc.2004-0164
Acknowledgements
The authors (KT, KAL, HML, LB and AKH) are members of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO-NMD).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All participants were informed about the purpose of the study and oral and written consent was obtained. The Institutional Ethics Committee of University Medical Center Göttingen approved the study (reference number 33/8/17).
Consent for publication
Not applicable.
Conflicts of interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tsaknakis, K., Jäckle, K., Lüders, K.A. et al. Reduced bone mineral density in adolescents with Duchenne Muscular Dystrophy (DMD) and scoliosis. Osteoporos Int 33, 2011–2018 (2022). https://doi.org/10.1007/s00198-022-06416-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-022-06416-9