Abstract
Summary
Patients with ankylosing spondylitis (AS) have impaired volumetric bone mineral density (vBMD) assessed with high-resolution peripheral computed tomography (HRpQCT). This first longitudinal HRpQCT study in AS shows that cortical and trabecular vBMD decreased at tibia and that signs of inflammation were associated with cortical bone loss at tibia and radius.
Introduction
Patients with ankylosing spondylitis (AS) have reduced volumetric bone mineral density (vBMD) in the peripheral skeleton assessed with high-resolution peripheral quantitative computed tomography (HRpQCT). The aims were to investigate longitudinal changes in vBMD, cortical area, and microarchitecture and to assess factors associated with changes in vBMD and cortical area in men with AS.
Methods
HRpQCT of radius and tibia was performed in 54 men with AS at baseline and after 5 years. Univariate and multivariable linear regression analyses were used.
Results
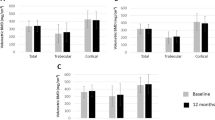
At tibia, there were significant decreases exceeding least significant changes (LSC) in cortical and trabecular vBMD, mean (SD) percent change −1.0 (1.9) and −2.7 (5.0) respectively (p<0.001). In multivariable regression analyses, increase in disease activity measured by ASDAS_CRP from baseline to follow-up was associated with decreases in cortical vBMD (β −0.86, 95% CI −1.31 to −0.41) and cortical area (β −1.66, 95% CI −3.21 to −0.10) at tibia. At radius, no changes exceeded LSC. Nonetheless, increase in ASDAS_CRP was associated with decreases in cortical vBMD, and high time-averaged ESR was associated with decreases in cortical area. Treatment with TNF inhibitor ≥ 4 years during follow-up was associated with increases in cortical vBMD and cortical area at tibia, whereas exposure to bisphosphonates was associated with increases in cortical measurements at radius. No disease-related variables or treatments were associated with changes in trabecular vBMD.
Conclusion
The findings in this first longitudinal HRpQCT study in patients with AS strengthen the importance of controlling disease activity to maintain bone density in the peripheral skeleton.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is a chronic, inflammatory disease mainly affecting the sacroiliac joints and the spine and is characterized by pathological spinal new bone formation [1]. Patients with AS also have higher prevalence of low bone mineral density (BMD) than controls, with prevalences varying between 4 and 58% in different cohorts [2]. Furthermore, patients with AS have almost doubled risk of vertebral fractures (VFs) compared to non-AS individuals in a meta-analysis which identified risk factors for prevalent VFs to be low BMD at the total hip and femoral neck, male sex, longer duration of AS, and radiographic AS-related spinal alterations [3]. The risk for non-VFs for patients with AS is less studied but was also increased [3].
The standard method to measure BMD is dual-energy x-ray absorptiometry (DXA) which assesses areal BMD (aBMD). Limitations with DXA are the inabilities to separate trabecular from cortical bone and to evaluate the microarchitecture. Bone strength is determined not only by BMD but also by the degree of mineralization, trabecular and cortical microarchitecture, and bone geometry [4]. With high-resolution peripheral quantitative computed tomography (HRpQCT), separate measurements of cortical and trabecular volumetric BMD (vBMD) as well as evaluation of the microarchitecture and geometry can be obtained at radius and tibia [5, 6]. HRpQCT-derived vBMD at tibia is highly correlated with bone strength at femur and lumbar spine [7], and HRpQCT measurements of tibia and radius have been shown to predict clinical fractures independent of aBMD in older men [8,9,10]. Previous HRpQCT studies have in comparisons with controls shown reduced cortical and total vBMD both at radius and tibia in AS patients [11] and at radius in non-radiographic axial spondyloarthritis (nr-axSpA) patients (tibia was not examined) [12]. Baseline data from the current cohort showed lower cortical vBMD at radius and lower trabecular vBMD at tibia than in controls [13]. To our knowledge, longitudinal HRpQCT data in AS patients have not been published before. Our aims with this prospective study were (1) to evaluate changes over 5 years in trabecular and cortical vBMD, cortical area, and trabecular microarchitecture at tibia and radius in a cohort of men with AS and (2) to assess factors associated with changes in vBMD and cortical area.
Patients and methods
Patients
Patients were recruited at baseline from three rheumatology clinics in western Sweden and were part of a larger study on osteoporosis [14] with inclusion criterion AS according to the modified New York criteria [15]. Exclusion criteria were psoriasis, inflammatory bowel disease, dementia, ongoing pregnancy, and difficulties in understanding the Swedish language. In total, 69 men, out of 204 patients with AS, were randomized in an age-adjusted algorithm to take part in the HRpQCT study [13]. These patients were invited to the 5-year follow-up. Approval by the regional ethics committee in Gothenburg was given both at baseline and at follow-up, and the study was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
The patients were assessed with the same methods at baseline and the 5-year follow-up. Questionnaires included medical history, lifestyle factors, and medications. Disease activity was assessed by the Bath AS Disease Activity Index (BASDAI) and the AS Disease Activity Score based on C-reactive protein (ASDAS_CRP) [16]. Physical function was assessed by the Bath AS Functional Index (BASFI) [16]. Physical examination included evaluation of back and hip mobility by the Bath AS Metrology Index (BASMI) [16]. The dose of prednisolone was estimated from the medical records and dichotomized into having used < or ≥ 450 mg prednisolone during follow-up (dose equivalent of 5 mg/day ≥ 3 months). Non-steroidal anti-inflammatory drug (NSAID) consumption during follow-up was quantified according to the recommendation by the Assessment of SpondyloArthritis international Society [17]. Use of tumor necrosis factor inhibitors (TNFi), conventional synthetic disease-modifying anti-rheumatic drugs (csDMARD), and bisphosphonates during follow-up was estimated from the medical records.
Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were analyzed by standard laboratory techniques. Time-averaged ESR and CRP for the follow-up period were calculated using results obtained from the medical records.
High-resolution peripheral quantitative computed tomography
vBMD, cortical area, and microarchitecture in the non-dominant distal radius and tibia were assessed using the same HRpQCT machine (Xtreme CT, Scanco Medical AG, Brüttisellen, Switzerland), software, and operator at baseline and follow-up. The standard protocol provided by the manufacturer was used. To reduce motion artefacts, the extremities were immobilized in a carbon-fiber shell. For measurement of the volume of interest, a reference line was manually placed at the end plate of the distal radius and tibia, and the first CT slice started 9.5 mm and 22.5 mm proximal of this line for radius and tibia, respectively. A total of 110 parallel slices (voxel size 82 μm) were obtained in the proximal direction at each measuring site, resulting in an approximately 9 mm 3D representation of the bone. Separation of cortical and trabecular regions was done automatically, and borders were thereafter inspected and corrected manually by the operator if necessary. An automated matching procedure was applied to ensure common region of interests for the repeated measurements [18]. Previously described methods to process the data [5, 19,20,21,22] were used to obtain the following parameters: trabecular vBMD (Tb.vBMD; mg/cm3), trabecular number (TbN; per mm), trabecular thickness (TbTh; mm), trabecular separation (TbSp; mm), cortical vBMD (Ct.vBMD mg/cm3), and cortical area (Ct.Ar; mm2). Tb.vBMD, Ct.vBMD, Ct.Ar, and TbN were measured directly, and the other parameters were derived. Tb.vBMD and Ct.vBMD assess bone density, and TbN, TbTh, and TbSp assess microarchitecture. Each scan was graded with a 5-point quality scale recommended by the manufacturer (1 = best, 5 = worst). Examinations with quality 1–3 and common region ≥ 80% were used for evaluation of microarchitecture (TbN, TbTh, and TbSp), whereas quality 1–4 was used for measurements of vBMD and Ct.Ar [23]. Coefficient of variation (CV) for repeated measurements from our clinic was as follows: for tibia Tb.vBMD, 0.5%; TbN, TbTh, and TbSp, 3.6%; Ct.vBMD, 0.3%; and Ct.Ar, 0.5% and for radius Tb.vBMD, 0.9%; TbN and TbSp, 4.8%; TbTh, 4.1%; Ct.vBMD, 0.6%; and Ct.Ar, 1.5%. The least significant change (LSC), change recognized with 95% confidence, was calculated: 2.77 × CV (https://www.iscd.org/resources/faqs/precision-assessment/).
The stability over time for the HRpQCT measurements was assessed with data from repeated scans of a phantom, containing five different densities, during the study period. The means and standard deviations from repeated yearly scans of each density were stable over time and are presented in Supplementary Figure 1.
Dual-energy x-ray absorptiometry
aBMD (g/cm2) was measured at the lumbar spine anteroposterior (AP) (vertebra L1–L4) and lateral (L2–L4) projection, the left hip (total hip and femoral neck), and the non-dominant forearm (total radius) using the same device (Hologic Discovery A, Hologic Inc., Bedford, MA, USA) at baseline and follow-up. CV for repeated measurements was 0.3 % for AP lumbar spine, 1.3 % for lateral lumbar spine, 0.6 % for total hip, 0.8 % for femoral neck, and 3.1 % for total radius. The T-score (compared to the young normal mean) and Z-score (compared to the age- and sex-matched mean) reference values were provided by the DXA scanner manufacturer and were not available for lumbar lateral spine.
Radiography
Lateral spinal radiographs were obtained at baseline and follow-up for grading of AS-related spinal alterations with modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). With mSASSS, anterior vertebral corners of the cervical and lumbar spine are graded between 0 and 3, and the total score ranges from 0 to 72 [24]. The radiographs were also assessed for vertebral fractures with the semiquantitative method Genant score. Based on the percentage of height reduction, vertebrae T4-L4 were graded 0 (normal), 1 (mild, 20–25 % height reduction), 2 (moderate, >25–40 % height reduction), or 3 (severe, > 40 % height reduction) [25]. All radiographs were assessed by the same radiologist with known chronological order but blinded to clinical data.
Statistics
Statistical analyses were performed using SPSS version 22 (IBM Corp., Armonk, NY, USA). Descriptive statistics are presented as numbers (percentage), mean (SD), or median (25th (Q1) to 75th (Q3) percentile). To compare continuous variables at baseline and follow-up, the paired t-test and the Wilcoxon signed rank test were used as appropriate. For categorical comparisons, McNemar’s test was used. A one-sided t-test was used to compare the Z-score in patients to the test value 0. Changes (∆-values) between baseline and follow-up were calculated. ∆-values in percent for vBMD, Ct.Ar, and aBMD were divided by time in months between examinations and multiplied by 60 to get a time-standardized value. Correlations were calculated using Spearman’s rank correlation coefficient (rs). To compare ∆-vBMD between groups, the Mann Whitney U-test was used. To analyze factors associated with ∆-vBMD and ∆-Ct.Ar, univariate and standard multivariable linear regression analyses were used. Variables with a univariate p-value ≤ 0.1 were considered for the multivariable models. All models were adjusted for age. Multicollinearity was checked for using variance inflation factor (VIF), and correlations between independent variables considered for the models were analyzed; high correlation was found for age and symptom duration, and age was kept in the models. High correlation was found for ∆-ASDAS and ∆-ESR. In this case and if a variable was part of another variable (ASDAS_CRP and BASDAI or ∆-ASDAS_CRP and ∆-CRP), the variable with the lowest univariate p-value was used. All models were initially adjusted for baseline HRpQCT measurement at the same site, and models with ∆-ASDAS_CRP were adjusted for baseline ASDAS_CRP. However, if that variable was not significant or did not affect the significance of the other independent variables, the variable was excluded. For ∆-cortical vBMD, more than five variables were eligible for the models. For cortical vBMD at radius, selection was based on lowest univariate p-value. For cortical vBMD at tibia, several eligible variables had similar univariate p-value, and some alternative models were tested. Variables were kept based on significance and contribution to the model with increases in the adjusted R2. Residual plots were assessed. There were very few missing data among the independent variables and none for the dependent variables. If missing data occurred, pairwise deletion was used. To test the robustness of the multivariable linear regression analyses, sensitivity analyses were performed; 10 % of the patients were randomly excluded, and then the multivariable regression analyses were repeated in the smaller sample size. Interactions and subgroups were not analyzed due to relatively small number of participants. We did not analyze factors associated with ∆-values for microarchitecture (TbN, TbTh, and TbSp) due to large CVs for these variables (presented in the method section “High-resolution peripheral computed tomography”), and since data from fewer patients were analyzed based on the higher quality of measurements needed for these variables. A p-value ≤ 0.05 was considered significant, and tests were two-tailed.
Results
Patients
Of 69 baseline patients, 57 (83%) were re-examined at the 5-year follow-up. However, motion artifacts affected radius image quality, resulting in 54 patients with quality 1–4 at both radius and tibia thereby assessed for ∆-vBMD and ∆-Ct.Ar, whereas 45 patients had quality 1–3, assessed for changes in microarchitecture (Fig. 1).
Characteristics at baseline and the 5-year follow-up are shown in Table 1. Of the 13 patients who used TNFi at baseline, 11 patients used TNFi throughout the follow-up time. Three patients started TNFi during the follow-up time.
Baseline age did not differ between participants in the 5-year follow-up vs those who declined participation (48±14 vs 48±18, p=0.94).
Changes in aBMD
The patients increased in aBMD at lumbar spine (AP and lateral projections) and total hip, also compared with the age- and sex-matched reference material (Z-scores). At femoral neck and total radius, aBMD decreased. The magnitude of the change in aBMD was similar to the age- and sex-matched references (Z-scores) (Table 1). However, the patients had significantly lower aBMD at these two sites than the reference population at follow-up, with Z-scores lower than 0 (p = 0.033 for femoral neck and p = 0.050 for radius).
Changes in vBMD, microarchitecture, and cortical area
At the 5-year follow-up, the 54 patients had decreased significantly at tibia in both Ct.vBMD and Tb.vBMD with mean (SD) percent change exceeding LSC (−1.0 (1.9), p<0.001 and −2.7 (5.0), p<0.001, respectively) (Table 2).
There were indications of worsening of the microarchitecture at tibia with decreases in TbN and increases in TbSp. At radius, TbN increased, whereas TbTh decreased. However, the changes were exceeded by the precision error (LSC) (Table 2).
Ct.Ar increased at radius, but the changes did not exceed LSC (Table 2).
Correlations between ∆-aBMD and ∆-vBMD were moderate to low (Table 3).
Factors associated with changes in vBMD and cortical area
Tibia
Univariate analyses (Supplementary Table 1)
Factors associated with ∆-Tb.vBMD were younger age and shorter symptom duration, which were associated with decreases in trabecular vBMD. According to scatterplots, large decreases in Tb.vBMD were mainly found in patients <40 years old (n = 18). Comparison of ∆-Tb.vBMD between patients < 40 years vs ≥ 40 years showed that younger patients decreased more than the older patients did (mean (SD) % change −5.8 (6.2) vs −1.1 (3.4), p=0.007).
For ∆-Ct.vBMD, older age, longer symptom duration, higher ∆-ASDAS_CRP, development of ≥ 1 new syndesmophyte, and use of prednisolone ≥ 450 mg during follow-up were associated with decreases.
For ∆-Ct.Ar, high baseline body mass index (BMI) and use of TNFi ≥ 4 years during follow-up were associated with increases.
Multivariable linear regression analyses (Table 4)
The factor independently associated with ∆-Tb.vBMD was age; younger age was associated with a decrease in trabecular vBMD.
Factors independently associated with decreases in ∆-Ct.vBMD and ∆-Ct.Ar were older age, higher ∆-ASDAS_CRP, and use of prednisolone ≥ 450 mg, whereas high baseline BMI and use of TNFi ≥ 4 years during follow-up were associated with increases.
Radius
Univariate analyses (Supplementary Table 2)
The factor associated with ∆-Tb.vBMD was ever smoking, predicting decreases.
For ∆-Ct.vBMD, older age, longer symptom duration, ever smoking, presence of ≥ 1 baseline syndesmophyte, and higher ∆-ASDAS_CRP were associated with decreases. Use of bisphosphonates during follow-up was associated with increases in ∆-Ct.vBMD, which was also found for ∆-Ct.Ar. Factors associated with a decrease in ∆-Ct.Ar were older age, longer symptom duration, higher ∆-ASDAS_CRP, and high time-averaged ESR.
Multivariable linear regression analyses (Table 4)
One variable was independently associated with ∆-Tb.vBMD. If a patient had ever smoked, it was associated with decreases in trabecular vBMD.
Factors independently associated with ∆-Ct.vBMD were older age and higher ∆-ASDAS_CRP associated with decreases and use of bisphosphonate during follow-up which was associated with increases. Analyses indicated possible multicollinearity for age and baseline syndesmophyte. Presence of baseline syndesmophyte was not significantly associated with ∆-Ct.vBMD in the model, and when excluded, the model improved and that model was kept.
For ∆-Ct.Ar, older age and high time-averaged ESR were associated with decreases, whereas use of bisphosphonates during follow-up was associated with increases. Additionally, a trend was found for high ∆-ASDAS_CRP being associated with decreases.
Sensitivity analyses for the multivariable linear regression analyses
The same independent factors were associated with the changes in vBMD and cortical area in the sensitivity analyses except for trabecular vBMD at tibia and cortical vBMD at radius. The association for younger age and decreases in trabecular vBMD at tibia was no longer significant. ∆-ASDAS_CRP was not significant but showed a trend of being associated with decreases in cortical vBMD at radius (Supplementary Table 3).
Baseline vBMD measurements in relation to ∆-vBMD
To study the relationship between baseline Tb.vBMD and ∆-Ct.vBMD and between baseline Ct.vBMD and ∆-Tb.vBMD, linear regression analyses were used. Results were similar for tibia and radius; in univariate analyses, high baseline Tb.vBMD was associated with increases in Ct.vBMD (Supplementary Table 1 and 2). However, including baseline Tb.vBMD in the multivariable analyses previously described, associations were no longer significant (p = 0.63 for tibia and p = 0.11 for radius). Baseline Ct.vBMD had no association with ∆-Tb.vBMD (Supplementary Table 1 and 2).
Development of vertebral fractures
There were six patients with VFs at baseline, and at the 5-year follow-up, no patient had developed new VFs (Table 1) or worsened in height reduction in previous VFs, according to the Genant score.
Discussion
We investigated 5-year changes in vBMD, cortical area, and microarchitecture at tibia and radius in men with AS and found mean decreases in cortical and trabecular vBMD exceeding LSC at tibia, whereas worsening of tibia trabecular microarchitecture did not exceed LSC. An increase in ASDAS_CRP and use of ≥ 450 mg prednisolone during follow-up were associated with decreases in cortical vBMD at tibia.
There are no previous longitudinal studies evaluating changes in HRpQCT measurements in patients with AS for comparison. Cross-sectional data are sparse but have shown patients with AS to differ from controls, especially in cortical bone. Haroon et al. found in their mixed gender AS cohort that AS was associated with lower cortical vBMD at tibia and radius, but not with trabecular vBMD or microarchitecture [11]. Also, in men and women with nr-axSpA, cortical vBMD, cortical area, and cortical thickness were reduced compared to controls at radius (tibia was not measured) [12]. In our baseline study on men with AS, patients had lower trabecular vBMD at tibia and lower cortical vBMD at radius than controls [13], whereas Caparbo et al. reported decreased trabecular vBMD, trabecular thickness, and trabecular separation at tibia and no differences at radius in AS patients vs controls [26].
We have no control group for longitudinal comparisons in this current study, but there are two longitudinal studies on general population assessing changes in HRpQCT measurements that we relate to for indirect comparison [27, 28]. These studies showed similar results with decrease in cortical vBMD for men > 70 years old at tibia and radius in the Canadian study [28] and at tibia in the Danish study [27], whereas trabecular vBMD was stable over time for all age groups. For a more in-depth comparison, the Canadian study presents mean annual percent change in bone parameters for baseline age groups in decades. One has to bear in mind though that we report percent change over 5 years, that other factors that might affect vBMD such as weight, diet, and physical exercise can differ, and that they have a rather low number of participants in each age group. Our patients are mean 48 years at baseline. In the age-span 40–49 years in the Canadian study, mean annual increases in cortical vBMD were 0.1 % and in trabecular vBMD 0.5 % at tibia. Trabecular changes were significant and exceeded LSC [28]. Our patients decreased significantly at tibia with mean 1.0 % in cortical vBMD and 2.7 % in trabecular vBMD over 5 years. At radius in the Canadian study, mean annual decrease in cortical vBMD was 0.1 %, whereas trabecular vBMD increased with 0.1 %. However, changes at radius were not significant, neither in this group nor in our group of patients [28]. Thus, the decrease in cortical vBMD at tibia found in our study seems to start earlier in AS men than in the general population. Moreover, the decreases in trabecular vBMD found at tibia in AS men, and especially in younger men, were not seen in the general population and might be related to the AS disease. However, we could not find any disease-related factor to be associated with the decrease in trabecular vBMD in the regression analyses, and Riggs et al. studied longitudinal changes in vBMD at radius and tibia using QCT in the general population and found decreases of trabecular vBMD throughout life [29]. The sole independent factor associated with changes in trabecular vBMD at tibia found in this present study was age. The level of explanation was low with a low adjusted R2. In the sensitivity analysis, age was no longer significantly associated with the changes in vBMD, and it is difficult to draw any firm conclusions why the patients decrease in trabecular vBMD.
For cortical vBMD at tibia, regression analyses showed that an increase in ASDAS_CRP from baseline to follow-up had a negative impact on the cortical vBMD, which was also found for cortical area at tibia. At radius, an increase in ASDAS_CRP affected cortical vBMD negatively and showed a trend to affect cortical area negatively. Also, a high time-averaged ESR was associated with a decrease in cortical area at radius. There are limited data regarding factors affecting the different bone compartments in AS patients. The negative associations between inflammation and changes in cortical measurements are in line with previously reported predominantly cortical alterations found in AS and nr-axSpA [11,12,13]. Further, Haroon et al found high ESR to correlate with lower cortical and trabecular tibia vBMD [11]. Previous longitudinal studies on AS patients assessing changes in aBMD by our group and others have shown persistent inflammation measured by ESR [30,31,32] or CRP [30, 33] to be associated with decreases in aBMD at the femoral neck [30,31,32,33] and lumbar spine [30, 32].
A high baseline BMI was found to predict increases in cortical vBMD and cortical area at tibia. High BMI and weight have been positively associated with aBMD in multiple studies, especially at the hip and lumbar spine [34]. That meta-analysis did not include radius. A study of elderly men and women found weight and BMI to affect aBMD at the weight-bearing sites femur and spine but not radius in men in line with our results [35]. One potential mechanism for BMI and weight affecting BMD at weight-bearing sites is through mechanical loading [36]. The association for BMI and HRpQCT measurements is less studied in men; however, one study found obese adults having higher cortical and trabecular vBMD as well as cortical area at both tibia and radius compared to normal weight adults [37].
Concerning treatments, use of TNFi during ≥ 4 years was associated with increase in tibia cortical bone. Several longitudinal studies have shown treatment with TNFi in patients with AS to increase aBMD at the lumbar spine [33, 38, 39], total hip [38, 39], and femoral neck in line with our results [40]. The underlying mechanisms for increases in BMD by TNFi are not fully elucidated, but we hypothesize that it is related to reduction in systemic inflammation. To our knowledge, the effect of TNFi on HRpQCT measurements has not been studied longitudinally in AS patients before.
A limited number of patients were exposed to bisphosphonates during follow-up. Nonetheless, such exposure was associated with increases in cortical vBMD and cortical area at radius. Data concerning the effect of bisphosphonates on BMD in AS are limited, and large randomized controlled studies on this matter are lacking. We have previously, in the total AS group, shown that exposure to bisphosphonates was associated with increases in aBMD at lumbar spine and hip [33]. Effects of alendronate on HRpQCT measurements are reported for postmenopausal women and thus not directly comparable to our AS men. Based on these studies, one would expect an impact of bisphosphonates also on tibia measurements [41, 42].
Corticosteroids have a negative impact on BMD and fractures [43], and use of ≥ 450 mg prednisolone during follow-up had a negative effect on tibia cortical bone, coherent with lower trabecular vBMD in prednisolone-treated patients compared with prednisolone-naïve patients in the cross-sectional study on nr-axSpA [12]. However, no firm conclusions of our results can be drawn based on only four patients with this medication.
Associations for disease-related variables and treatments with changes in bone parameters somewhat differ between radius and tibia in this study. The precision of the measurements at radius is lower than at tibia, shown in larger LSC. The greater measurement error for radius could possibly affect the results in this rather small sample size with few patients exposed to the treatments. Further, the explanatory level of the models is at best 54 % (for tibia cortical vBMD). With much of the variation unexplained, it is difficult to determine why the patients decrease in vBMD at tibia but not at radius.
The precision errors for the assessment of microarchitecture are by far exceeding the observed changes in trabecular separation, trabecular thickness, and trabecular number. This makes it difficult to draw firm conclusions on the differences found at radius and tibia.
This study is performed on men with longstanding AS, and whether results apply to women and patients with short symptom duration need to be elucidated.
Limitation with this study is the lack of a control group, although the measurements of the machine were stable over time. Another limitation is a relatively small number of patients, which hampers precision of change over time, and preclude interaction analyses and subgroup analyses of for example those exposed to different treatments. In addition, multiple statistical tests were performed in the study, and the increase in familywise error rate across the statistical analyses was not controlled, which increases the risk of false-positive results. We consider this study exploratory, and our results need to be confirmed in independent studies. We also lack data about physical activity during follow-up, a factor that could possibly influence measurements at tibia. However, we do have information about baseline occupation, and when patients were categorized into blue-collar workers (manual labor), white-collar workers (less physical activity), or no work, there were no associations between changes in vBMD or cortical area at tibia and type of occupation (data not shown). Strengths are the long follow-up time with well-characterized patients in this first longitudinal HRpQCT study in men with AS.
In conclusion, HRpQCT is not a method for use in clinical practice yet, however, to improve the knowledge about the complex mechanism of skeletal involvement in AS, information from HRpQCT measurements is valuable to further understand how different bone compartments are affected. We have here shown that over 5 years, AS men decreased in trabecular and cortical vBMD at tibia and that inflammation-related factors and medications were associated with changes in cortical bone. We could not find any significant change in vBMD at radius, and changes in microarchitecture did not exceed the precision of the method. No disease-related variables or treatments were found to be associated with changes in trabecular vBMD. The findings strengthen the importance of controlling disease activity in patients with AS to maintain bone density also in the peripheral skeleton.
Data availability
The data sets generated and/or analyzed during the current study are not publicly available due to the General Data Protection Regulation (GDPR). Researchers with a specific question regarding the study are encouraged to contact the corresponding author (AD).
Change history
02 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00198-021-06156-2
References
Braun J, Sieper J (2007) Ankylosing spondylitis. Lancet (London, England) 369(9570):1379–1390. https://doi.org/10.1016/s0140-6736(07)60635-7
Fitzgerald GE, O’Shea FD (2017) The fascinating paradox of osteoporosis in axial spondyloarthropathy. J Rheumatol 44(12):1767–1776. https://doi.org/10.3899/jrheum.170051
Pray C, Feroz NI, Nigil Haroon N (2017) Bone mineral density and fracture risk in ankylosing spondylitis: a meta-analysis. Calcif Tissue Int 101(2):182–192. https://doi.org/10.1007/s00223-017-0274-3
Fonseca H, Moreira-Gonçalves D, Coriolano H-JA, Duarte JA (2014) Bone quality: the determinants of bone strength and fragility. Sports Med 44(1):37–53. https://doi.org/10.1007/s40279-013-0100-7
Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90(12):6508–6515. https://doi.org/10.1210/jc.2005-1258
Cheung AM, Adachi JD, Hanley DA, Kendler DL, Davison KS, Josse R, Brown JP, Ste-Marie LG, Kremer R, Erlandson MC, Dian L, Burghardt AJ, Boyd SK (2013) High-resolution peripheral quantitative computed tomography for the assessment of bone strength and structure: a review by the canadian bone strength working group. Curr Osteoporos Rep 11(2):136–146. https://doi.org/10.1007/s11914-013-0140-9
Kroker A, Plett R, Nishiyama KK, McErlain DD, Sandino C, Boyd SK (2017) Distal skeletal tibia assessed by HR-pQCT is highly correlated with femoral and lumbar vertebra failure loads. J Biomech 59:43–49. https://doi.org/10.1016/j.jbiomech.2017.05.011
Langsetmo L, Peters KW, Burghardt AJ, Ensrud KE, Fink HA, Cawthon PM, Cauley JA, Schousboe JT, Barrett-Connor E, Orwoll ES (2018) Volumetric bone mineral density and failure load of distal limbs predict incident clinical fracture independent HR-pQCT BMD and failure load predicts incident clinical fracture of frax and clinical risk factors among older men. J Bone Miner Res 33(7):1302–1311. https://doi.org/10.1002/jbmr.3433
Ohlsson C, Sundh D, Wallerek A, Nilsson M, Karlsson M, Johansson H, Mellstrom D, Lorentzon M (2017) Cortical bone area predicts incident fractures independently of areal bone mineral density in older men. J Clin Endocrinol Metab 102(2):516–524. https://doi.org/10.1210/jc.2016-3177
Szulc P, Boutroy S, Chapurlat R (2018) Prediction of fractures in men using bone microarchitectural parameters assessed by high-resolution peripheral quantitative computed tomography-the prospective STRAMBO study. J Bone Miner Res 33(8):1470–1479. https://doi.org/10.1002/jbmr.3451
Nigil Haroon N, Szabo E, Raboud JM, McDonald-Blumer H, Fung L, Josse RG, Inman RD, Cheung AM (2015) Alterations of bone mineral density, bone microarchitecture and strength in patients with ankylosing spondylitis: a cross-sectional study using high-resolution peripheral quantitative computerized tomography and finite element analysis. Arthritis Res Ther 17:377. https://doi.org/10.1186/s13075-015-0873-1
Neumann A, Haschka J, Kleyer A, Schuster L, Englbrecht M, Berlin A, Figueiredo CP, Simon D, Muschitz C, Kocijan R, Resch H, Rech J, Schett G (2018) Cortical bone loss is an early feature of nonradiographic axial spondyloarthritis. Arthritis Res Ther 20(1):202. https://doi.org/10.1186/s13075-018-1620-1
Klingberg E, Lorentzon M, Gothlin J, Mellstrom D, Geijer M, Ohlsson C (2013) Bone microarchitecture in ankylosing spondylitis and the association with bone mineral density, fractures, and syndesmophytes. Arthritis Res Ther 15:R179
Klingberg E, Lorentzon M, Mellstrom D, Geijer M, Gothlin J, Hilme E, Hedberg M, Carlsten H, Forsblad-d ' Elia H (2012) Osteoporosis in ankylosing spondylitis - prevalence, risk factors and methods of assessment. Arthritis Res Ther 14(3):12. https://doi.org/10.1186/ar3833
van der Linden S, Valkenburg HA, Cats A (1984) Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 27(4):361–368
Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R, Dougados M, Hermann KG, Landewé R, Maksymowych W, van der Heijde D (2009) The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 68(Suppl 2):ii1–ii44. https://doi.org/10.1136/ard.2008.104018
Dougados M, Simon P, Braun J, Burgos-Vargas R, Maksymowych WP, Sieper J, Heijde D (2011) ASAS recommendations for collecting, analysing and reporting NSAID intake in clinical trials/epidemiological studies in axial spondyloarthritis. Ann Rheum Dis 70:249–251. https://doi.org/10.1136/ard.2010.133488
MacNeil JA, Boyd SK (2008) Improved reproducibility of high-resolution peripheral quantitative computed tomography for measurement of bone quality. Med Eng Phys 30(6):792–799. https://doi.org/10.1016/j.medengphy.2007.11.003
Amin S, Khosla S (2012) Sex- and age-related differences in bone microarchitecture in men relative to women assessed by high-resolution peripheral quantitative computed tomography. J Osteoporos 2012:129760–129766. https://doi.org/10.1155/2012/129760
Laib A, Häuselmann HJ, Rüegsegger P (1998) In vivo high resolution 3D-QCT of the human forearm. Technol Health Care 6(5-6):329–337
Sundh D, Mellström D, Nilsson M, Karlsson M, Ohlsson C, Lorentzon M (2015) Increased Cortical Porosity in Older Men With Fracture. J Bone Miner Res 30(9):1692–1700. https://doi.org/10.1002/jbmr.2509
Sundh D, Nilsson AG, Nilsson M, Johansson L, Mellström D, Lorentzon M (2017) Increased cortical porosity in women with hip fracture. J Intern Med 281(5):496–506. https://doi.org/10.1111/joim.12587
Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S (2012) Visual grading of motion induced image degradation in high resolution peripheral computed tomography: impact of image quality on measures of bone density and micro-architecture. Bone 50(1):111–118. https://doi.org/10.1016/j.bone.2011.10.003
Creemers MC, Franssen MJ, van ' t Hof MA, Gribnau FW, van de Putte LB, van Riel PL (2005) Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 64(1):127–129. https://doi.org/10.1136/ard.2004.020503
Genant H, Wu C, Kuijk C, Nevitt M (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148. https://doi.org/10.1002/jbmr.5650080915
Caparbo VF, Furlam P, Saad CGS, Alvarenga JC, Aubry-Rozier B, Hans D, de Brum-Fernandes AJ, Pereira RMR (2019) Assessing bone impairment in ankylosing spondylitis (AS) using the trabecular bone score (TBS) and high-resolution peripheral quantitative computed tomography (HR-pQCT). Bone 122:8–13. https://doi.org/10.1016/j.bone.2019.01.024
Shanbhogue VV, Brixen K, Hansen S (2016) Age- and sex-related changes in bone microarchitecture and estimated strength: a three-year prospective study using HRpQCT. J Bone Miner Res 31(8):1541–1549. https://doi.org/10.1002/jbmr.2817
Burt LA, Hanley DA, Boyd SK (2017) Cross-sectional versus longitudinal change in a prospective HR-pQCT study. J Bone Miner Res 32(7):1505–1513. https://doi.org/10.1002/jbmr.3129
Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S (2008) A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res 23(2):205–214. https://doi.org/10.1359/jbmr.071020
Gratacos J, Collado A, Pons F, Osaba M, Sanmarti R, Roque M (1999) Significant loss of bone mass in patients with early, active ankylosing spondylitis: a follow-up study. Arthritis Rheum 42(11):2319–2324. https://doi.org/10.1002/1529-0131(199911)42:11<2319::Aid-Anr9>3.0.Co;2-g
Maillefert JF, Aho LS, El Maghraoui A, Dougados M, Roux C (2001) Changes in bone density in patients with ankylosing spondylitis: a two-year follow-up study. Osteoporos Int 12(7):605–609. https://doi.org/10.1007/s001980170084
Wang DM, Zeng QY, Chen SB, Gong Y, Hou ZD, Xiao ZY (2015) Prevalence and risk factors of osteoporosis in patients with ankylosing spondylitis: a 5-year follow-up study of 504 cases. Clin Exp Rheumatol 33(4):465–470
Deminger A, Klingberg E, Lorentzon M, Geijer M, Göthlin J, Hedberg M, Rehnberg E, Carlsten H, Jacobsson LT, Forsblad-d’Elia H (2017) Which measuring site in ankylosing spondylitis is best to detect bone loss and what predicts the decline: results from a 5-year prospective study. Arthritis Res Ther 19(1):273. https://doi.org/10.1186/s13075-017-1480-0
Papaioannou A, Kennedy CC, Cranney A, Hawker G, Brown JP, Kaiser SM, Leslie WD, O ' Brien CJ, Sawka AM, Khan A, Siminoski K, Tarulli G, Webster D, McGowan J, Adachi JD (2009) Risk factors for low BMD in healthy men age 50 years or older: a systematic review. Osteoporos Int 20(4):507–518. https://doi.org/10.1007/s00198-008-0720-1
Felson DT, Zhang Y, Hannan MT, Anderson JJ (1993) Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res 8(5):567–573. https://doi.org/10.1002/jbmr.5650080507
Morseth B, Emaus N, Jørgensen L (2011) Physical activity and bone: the importance of the various mechanical stimuli for bone mineral density. A review. Nor Epidemiol 20(2). https://doi.org/10.5324/nje.v20i2.1338
Evans AL, Paggiosi MA, Eastell R, Walsh JS (2015) Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J Bone Miner Res 30(5):920–928. https://doi.org/10.1002/jbmr.2407
Haroon NN, Sriganthan J, Al Ghanim N, Inman RD, Cheung AM (2014) Effect of TNF-alpha inhibitor treatment on bone mineral density in patients with ankylosing spondylitis: a systematic review and meta-analysis. Semin Arthritis Rheum 44(2):155–161. https://doi.org/10.1016/j.semarthrit.2014.05.008
van der Weijden MA, van Denderen JC, Lems WF, Nurmohamed MT, Dijkmans BA, van der Horst-Bruinsma IE (2016) Etanercept increases bone mineral density in ankylosing spondylitis, but does not prevent vertebral fractures: results of a prospective observational cohort study. J Rheumatol 43(4):758–764. https://doi.org/10.3899/jrheum.150857
Li H, Li Q, Chen X, Ji C, Gu J (2015) Anti-tumor necrosis factor therapy increased spine and femoral neck bone mineral density of patients with active ankylosing spondylitis with low bone mineral density. J Rheumatol 42(8):1413–1417. https://doi.org/10.3899/jrheum.150019
Burghardt AJ, Kazakia GJ, Sode M, de Papp AE, Link TM, Majumdar S (2010) A longitudinal HR-pQCT study of alendronate treatment in postmenopausal women with low bone density: relations among density, cortical and trabecular microarchitecture, biomechanics, and bone turnover. J Bone Miner Res 25(12):2558–2571. https://doi.org/10.1002/jbmr.157
Seeman E, Delmas PD, Hanley DA, Sellmeyer D, Cheung AM, Shane E, Kearns A, Thomas T, Boyd SK, Boutroy S, Bogado C, Majumdar S, Fan M, Libanati C, Zanchetta J (2010) Microarchitectural deterioration of cortical and trabecular bone: differing effects of denosumab and alendronate. J Bone Miner Res 25(8):1886–1894. https://doi.org/10.1002/jbmr.81
van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13(10):777–787. https://doi.org/10.1007/s001980200108
Acknowledgements
We wish to thank all the patients that participated in the study.
Funding
Open access funding provided by University of Gothenburg. This study was supported by grants from the Health and Medical Care Executive Board of the Västra Götaland (VGFOUREG-383071, VGFOUREG-564511, VGFOUREG-754661), the Swedish Research Council (2016-02035 to HFd’E, 2017-02229 to ML) Rune and Ulla Amlövs foundation for Rheumatology Research, Göteborg’s Association Against Rheumatism, the Swedish Association Against Rheumatism, the Swedish Society of Medicine, the Göteborg Medical Society, the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG-141111 to HFd’E, ALFGBG-430851 to HFd’E, ALFVLL-640251 to HFd’E, ALFGBG-716051 to ML), Controlling Chronic Inflammatory Diseases with Combined Efforts (COMBINE), and the Margareta Rheuma Research Foundation and by a grant from IngaBritt och Arne Lundbergs Forskningsstiftelse (to ML).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the regional ethics committee in Gothenburg, Sweden (reference numbers Dnr: 597-08 and Dnr: 690-13) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Conflicts of interest
EK has received Advisory Board Fees from Novartis, lecturing fees from Lilly, and an unrestricted grant from Roche, outside the submitted work. ML has received lecturing fees from Amgen, Meda, Jansen-Cilag AB, Consilient Health, and Lilly, consulting fees from Radius Health and UCB Pharma, and a grant from BioGaia AB, outside the submitted work. LTHJ has received Advisory Board Fees from Novartis, Celgene, and Eli Lilly, outside the submitted work. HFd’E has received Advisory Board Fees from Sandoz, AbbVie, and Novartis and an unrestricted grant from Novartis, outside the submitted work. AD, MH, and HC report no competing interests.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The title of the PDF version of the article unfortunately contains an error due to a typesetting mistake.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Deminger, A., Klingberg, E., Lorentzon, M. et al. Factors associated with changes in volumetric bone mineral density and cortical area in men with ankylosing spondylitis: a 5-year prospective study using HRpQCT.. Osteoporos Int 33, 205–216 (2022). https://doi.org/10.1007/s00198-021-06049-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-021-06049-4