Abstract
Summary
Bisphosphonates can inhibit osteoclast-mediated bone resorption, prevent bone loss, and reduce the risk of osteoporotic fractures. Our meta-analysis of studies shows that early bisphosphonate administration after SCI was safe and beneficial to the BMD of the total hip and lumbar spine at 12 months.
Introduction
Rapid bone loss in the early stages of spinal cord injury (SCI) leads to an increased risk of osteoporotic fracture. A meta-analysis was conducted to assess the efficacy and safety of bisphosphonates for the treatment of osteoporosis after SCI.
Methods
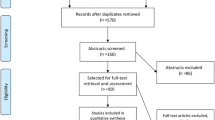
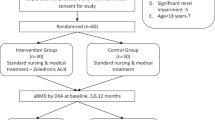
A literature search of the PubMed, EMBASE, Cochrane Library, and Web of Science databases identified nine randomized controlled trials with 206 individuals. This meta-analysis was performed using a random-effects model. The primary outcome was the percent change in bone mineral density (BMD) of the total hip, distal femur, and lumbar spine from baseline to 12 months. Bone turnover markers were secondary outcomes. The incidences of adverse events were assessed in order to evaluate safety.
Results
There were significant differences in BMD of the total hip and lumbar spine or serum C-terminal telopeptide between the bisphosphonate and control groups but no difference in adverse events. The percent change in BMD of the distal femur and serum type 1 procollagen N-terminal peptide from baseline to 12 months was not superior in the treatment groups. Osteoclast-mediated bone resorption was inhibited by bisphosphonate administration. Subgroup analyses of participants treated with zoledronate at different sites revealed a beneficial effect on BMD of the total hip and lumbar spine but not the distal femur.
Conclusion
Early bisphosphonate administration after SCI was safe and beneficial to the BMD of the total hip and lumbar spine at 12 months.



Similar content being viewed by others
References
Xie J, Deng X, Feng Y, Cao N, Zhang X, Fang F, Zhang S, Feng Y (2018) Early intradural microsurgery improves neurological recovery of acute spinal cord injury: a study of 87 cases. J Neurorestoratol 6(1):152–157. https://doi.org/10.26599/jnr.2018.9040014
Kang Y, Ding H, Zhou H, Wei Z, Liu L, Pan D, Feng S (2018) Epidemiology of worldwide spinal cord injury: a literature review. J Neurorestoratol 6(1):1–9. https://doi.org/10.2147/jn.S143236
Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H (2004) Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 34(5):869–880. https://doi.org/10.1016/j.bone.2004.01.001
Garland DE (1992) Osteoporosis after spinal cord injury. J Orthop Res 10:371–378
Minoshima M, Kikuta J, Omori Y, Seno S, Suehara R, Maeda H, Matsuda H, Ishii M, Kikuchi K (2019) In vivo multicolor imaging with fluorescent probes revealed the dynamics and function of osteoclast proton pumps. ACS Cent Sci 5(6):1059–1066. https://doi.org/10.1021/acscentsci.9b00220
Jiang SD, Dai LY, Jiang LS (2006) Osteoporosis after spinal cord injury. Osteoporos Int 17(2):180–192. https://doi.org/10.1007/s00198-005-2028-8
Soleyman-Jahi S, Yousefian A, Maheronnaghsh R, Shokraneh F, Zadegan SA, Soltani A, Hosseini SM, Vaccaro AR, Rahimi-Movaghar V (2018) Evidence-based prevention and treatment of osteoporosis after spinal cord injury: a systematic review. Eur Spine J 27(8):1798–1814. https://doi.org/10.1007/s00586-017-5114-7
Fleisch H (1993) New bisphosphonates in osteoporosis. Osteoporos Int 3:S15–S22
Brodowicz T, O’Byrne K, Manegold C (2012) Bone matters in lung cancer. Ann Oncol 23(9):2215–2222. https://doi.org/10.1093/annonc/mds009
Shapiro CL, Moriarty JP, Dusetzina S, Himelstein AL, Foster JC, Grubbs SS, Novotny PJ, Borah BJ (2017) Cost-effectiveness analysis of monthly zoledronic acid, zoledronic acid every 3 months, and monthly denosumab in women with breast cancer and skeletal metastases. J Clin Oncol 35(35):3949–3955. https://doi.org/10.1200/JCO.2017.73.7437
Moher D, Liberati A, Tetzlaff J (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Jokstad A (2017) Cochrane Collaboration Systematic Reviews may be based on trials not approved by a research ethics committee. Clin Exp Dent Res 3(5):179–182
Chang KV, Chen SY, Chen WS, Tu YK, Chien KL (2012) Comparative effectiveness of focused shock wave therapy of different intensity levels and radial shock wave therapy for treating plantar fasciitis: a systematic review and network meta-analysis. Arch Phys Med Rehabil 93:1259–1268. https://doi.org/10.1016/j.apmr.2012.02.023
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13. https://doi.org/10.1186/1471-2288-5-13
Li T, Ma J, Zhao T, Gao F, Sun W (2019) Application and efficacy of extracorporeal shockwave treatment for knee osteoarthritis: a systematic review and meta-analysis. Exp Ther Med 18(4):2843–2850. https://doi.org/10.3892/etm.2019.7897
Thorlund K, Imberger G, Johnston BC, Walsh M, Awad T, Thabane L, Gluud C, Devereaux PJ, Wetterslev J (2012) Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS One 7(7):e39471
Firth J, Teasdale SB, Allott K, Siskind D, Marx W, Cotter J, Veronese N, Schuch F, Smith L, Solmi M, Carvalho AF, Vancampfort D, Berk M, Stubbs B, Sarris J (2019) The efficacy and safety of nutrient supplements in the treatment of mental disorders: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry 18(3):308–324. https://doi.org/10.1002/wps.20672
Bradburn MJ, Deeks JJ, Berlin JA (2007) Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 26(1):53–77
Park HR, Kim JH, Lee D, Jo HG (2019) Cangfu daotan decoction for polycystic ovary syndrome: a protocol of systematic review and meta-analysis. Medicine (Baltimore) 98(39):e17321. https://doi.org/10.1097/MD.0000000000017321
Palmer SC, Nistor I, Craig JC, Pellegrini F, Messa P, Tonelli M, Covic A, Strippoli GF (2013) Cinacalcet in patients with chronic kidney disease: a cumulative meta-analysis of randomized controlled trials. PLoS Med 10(4):e1001436. https://doi.org/10.1371/journal.pmed.1001436
Bauman WA, Wecht JM, Kirshblum S, Spungen AM, Morrison N, Cirnigliaro C, Schwartz E (2005) Effect of pamidronate administration on bone in patients with acute spinal cord injury. J Rehabil Res Dev 42(3):305–313. https://doi.org/10.1682/JRRD.2004.05.0062
Bubbear JS, Gall A, Middleton FR, Ferguson-Pell M, Swaminathan R, Keen RW (2011) Early treatment with zoledronic acid prevents bone loss at the hip following acute spinal cord injury. Osteoporos Int 22(1):271–279. https://doi.org/10.1007/s00198-010-1221-6
Gilchrist NL, Frampton CM, Acland RH, Nicholls MG, March RL, Maguire P, Heard A, Reilly P, Marshall K (2007) Alendronate prevents bone loss in patients with acute spinal cord injury: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 92(4):1385–1390. https://doi.org/10.1210/jc.2006-2013
Goenka S, Sethi S, Pandey N, Joshi M, Jindal R (2018) Effect of early treatment with zoledronic acid on prevention of bone loss in patients with acute spinal cord injury: a randomized controlled trial. Spinal Cord 56(12):1207–1211. https://doi.org/10.1038/s41393-018-0195-7
Moran De Brito CM, Battistella LR, Saito ET, Sakamoto H (2005) Effect of alendronate on bone mineral density in spinal cord injury patients: a pilot study. Spinal Cord 43(6):341–348. https://doi.org/10.1038/sj.sc.3101725
Oleson CV, Marino RJ, Formal CS, Modlesky CM, Leiby BE (2020) The effect of zoledronic acid on attenuation of bone loss at the hip and knee following acute traumatic spinal cord injury: a randomized-controlled study. Spinal Cord 58:921–929. https://doi.org/10.1038/s41393-020-0431-9
Schnitzer TJ, Kim K, Marks J, Yeasted R, Simonian N, Chen D (2016) Zoledronic acid treatment after acute spinal cord injury: results of a randomized, placebo-controlled pilot trial. Pm r 8(9):833–843. https://doi.org/10.1016/j.pmrj.2016.01.012
Shapiro J, Smith B, Beck T, Ballard P, Dapthary M, BrintzenhofeSzoc K, Caminis J (2007) Treatment with zoledronic acid ameliorates negative geometric changes in the proximal femur following acute spinal cord injury. Calcif Tissue Int 80(5):316–322. https://doi.org/10.1007/s00223-007-9012-6
Varghese SM, Chandy BR, Thomas R, Tharion G (2016) Effect of zoledronic acid on osteoporosis after chronic spinal cord injury: a randomized controlled trial. Crit Rev Phys Rehabil Med 28(1-2):85–93. https://doi.org/10.1615/CritRevPhysRehabilMed.2016018768
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 7041(312):1254–1259. https://doi.org/10.1136/bmj.312.7041.1254
Garland DE, Adkins RH, Kushwaha V, Stewart C (2004) Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med 27(3):202–206. https://doi.org/10.1080/10790268.2004.11753748
Bauman WA, Cirnigliaro CM, La Fountaine MF, Martinez LA, Kirshblum SC, Spungen AM (2015) Zoledronic acid administration failed to prevent bone loss at the knee in persons with acute spinal cord injury: an observational cohort study. J Bone Miner Metab 33(4):410–421. https://doi.org/10.1007/s00774-014-0602-x
Cranney A, Wells G, Willan A, Griffith L, Zytaruk N, Robinson V, Black D, Adachi JD, Shea B, Tugwell P, Guyatt G (2002) Metaanalyses of therapies for postmenopausal osteoporosis. II. Metaanalysis of alendronate for the treatment of postmenopausal women. Endocr Rev 23:508–516
Nance PW, Schryvers O, Leslie W, Ludwig S, Krahn J, Uebelhart D (1999) Intravenous pamidronate attenuates bone density loss after acute spinal cord injury. Arch Phys Med Rehabil 3(80):243–251
Zehnder Y, Risi S, Michel D, Knecht H, Perrelet R, Kraenzlin M, Zäch GA, Lippuner K (2004) Prevention of bone loss in paraplegics over 2 years with alendronate. J Bone Miner Res 19(7):1067–1074. https://doi.org/10.1359/JBMR.040313
Acknowledgments
The authors would like to thank the Department of Spine Surgery, Beijing Bo’ai Hospital, China Rehabilitation Research Center for the generous support and the International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding
Projects of China Rehabilitation Research Center (NO.2017ZX-02) and Capital’s Funds for Health Improvement and Research (NO.2018-3-6012) funded this submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Code availability
Analyses were conducted using Review Manager 5.3 software (https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Y., Wang, F. & Zhang, Z. The efficacy and safety of bisphosphonate analogs for treatment of osteoporosis after spinal cord injury: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int 32, 1117–1127 (2021). https://doi.org/10.1007/s00198-020-05807-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05807-0