Abstract
Denosumab discontinuation is associated with a rapid increase in bone resorption and a decrease in bone mineral density. Spontaneous vertebral fractures may occur as a side effect of the rebound of bone resorption. Cases of rebound-linked hypercalcemia have also been described, moderate in women with osteoporosis and breast cancer and severe in children receiving oncological doses of denosumab. We report the case of an adult woman with primary hyperparathyroidism and moderate hypercalcemia, treated with denosumab for osteoporosis, who developed severe hypercalcemia and spontaneous vertebral fractures (SVFs) after denosumab discontinuation. An 86-year-old woman with densitometric osteoporosis was treated for 3 years with 60 mg of subcutaneous denosumab every 6 months. She was known to have primary hyperparathyroidism, with a serum albumin-corrected calcium of 2.82 mmol/l (NV 2.15–2.5) at the end of denosumab effect. Nine months after the last denosumab injection, she was hospitalized due to worsening overall health. Clinical evaluation revealed severe hypercalcemia (calcium 3.35 mmol/l). Very high values of bone turnover markers (BTMs) suggested a rebound effect due to denosumab discontinuation. An X-ray showed multiple new SVFs. After injection of denosumab 60 mg, serum calcium rapidly decreased and BTMs were dramatically reduced. A surgical approach by minimally invasive parathyroidectomy allowed for definite resolution of hyperparathyroidism and hypercalcemia. This case suggests that hypercalcemia can be a side consequence of denosumab discontinuation, which can become severe when other causes of hypercalcemia, such as primary hyperparathyroidism, are present.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Denosumab, a fully human monoclonal antibody that blocks the receptor activator of nuclear factor k-B ligand (RANKL), is a potent anti-resorptive osteoporosis treatment. Due to its reversible mode of action, bone turnover markers (BTMs) increase rapidly after its discontinuation, accompanied by a quick loss of bone mineral density (BMD) [1]. Spontaneous vertebral fractures (SVFs) have been described in this situation and a causative link to the rebound of bone resorption has been suggested [2]. Cases of rebound-linked hypercalcemia have also been described after denosumab discontinuation, from completely asymptomatic [3, 4] to severe clinical consequences [5,6,7,8,9,10,11,12,13]. Only two cases have been described in patients with moderate symptoms treated with denosumab for osteoporosis [4, 14]; all other patients had received denosumab at high (oncological) and/or frequent (minimum every 3 months) dosages.
We report the case of a patient who developed severe acute hypercalcemia after discontinuation of denosumab 60 mg given every 6 months for osteoporosis in the context of known hyperparathyroidism.
Case report
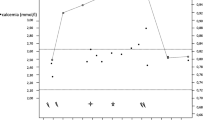
An 86-year-old woman with hypercalcemia was diagnosed in 2013 in the context of primary hyperparathyroidism by her endocrinologist. At that time, she refused the proposed surgical treatment. Due to densitometric osteoporosis (lumbar spine T-score, − 6.0 SD; femoral neck T-score, − 4.5 SD), denosumab 60 mg was given subcutaneously every 6 months from 2013 to October 2016 (last injection) with good but insufficient effect (04.2015: lumbar spine T-score, − 5.4 SD; femoral neck T-score, − 3.9 SD). The reason for the treatment discontinuation is unknown. In April 2017, at the end of denosumab efficacy (6 months post last injection), serum albumin-corrected calcium (referred as calcium from here on) level was 2.82 mmol/l (NV 2.15–2.50) and serum parathyroid hormone (PTH) level was 24.2 pmol/l (NV 1.3–9.3) (Table 1). Treatment with cinacalcet 30 mg/day was introduced and calcium decreased to 2.51 mmol/l 1 month later, suggesting good control of hypercalcemia, but the cinacalcet was lowered first and finally discontinued by the patient due to digestive intolerance. It was reintroduced mid-July 2017 following a re-increase in calcemia (calcium 3.53 mmol/l). At the end of July 2017, the patient was hospitalized due to weight loss (5 kg, 15% body weight), malnutrition, and poor health status (asthenia, confusion). Clinical evaluation revealed severe hypercalcemia (calcium 3.35 mmol/l), with lower PTH under cinacalcet (10.0 pmol/l). The serum 25-OH vitamin D level was 77 nmol/l (NV 50–75). Initial treatment with hydration and intranasal calcitonin only partially corrected calcium to 2.57 mmol/l, and then, despite cinacalcet maintaining low PTH (6.5 pmol/l) calcemia increased again (calcium 3.11 mmol/l). She was at that point referred to our center. Type I collagen c-terminal telopeptide (CTX) was high at 1777 ng/l (NV < 573), despite mild renal dysfunction (creatinine 97 μmol/l, NV 44–80 μmol/l; eGFR 50 ml/min/1.73 m2) suggesting a rebound effect due to denosumab discontinuation. A spinal X-ray showed three new SVFs. Denosumab treatment was restored, and after a single 60 mg injection, serum calcium rapidly decreased to 2.63 mmol/l. CTX decreased dramatically to 122 ng/l. With the resumption of denosumab injections (60 mg twice a year), calcemia remained stable between 2.60 and 2.80 mmol/l, without any other treatment. PTH levels increased when calcemia was close to normal values. The patient finally accepted surgical treatment for a parathyroid adenoma identified by neck ultrasound and 99mTc-MIBI SPECT/CT scintigraphy. Surgery resulted in calcium normalization in the presence of denosumab treatment (Table 1).
Discussion
This case suggests that, in patients with known hypercalcemia due to primary hyperparathyroidism, high bone remodeling induced by denosumab discontinuation can elicit acute hypercalcemia with severe clinical consequences.
Denosumab discontinuation induces a rebound effect, with BTMs rapidly increasing over baseline values after its discontinuation, a quick loss of BMD, and an increased risk of SVFs. During this period, hypercalcemia has also been recognized as a consequence of high bone resorption, with most cases described in children [3, 5,6,7,8,9,10,11, 15]. Affected children were 4 months to 15 years old, received 120 mg of denosumab (or weight-adapted dosage) for 7 to 42 months, and developed hypercalcemia from 7 weeks to 7 months after the last denosumab injection (Table 2). In seven cases, there were dramatic consequences (hospitalization, bone pain, dehydration with renal insufficiency, bradycardia). In order to normalize calcium values, different management strategies were applied, all including hydration and i.v. bisphosphonates (pamidronate, zoledronate), as well as calcitonin, corticosteroids, furosemide, or, in one case, resumption of denosumab [5]. In all cases, multiple treatments, given for up to 4 weeks, were required to control rebound hypercalcemia [4,5,6,7, 10,11,12,13, 15].
As the first cases were reported in children, it has been suggested that rebound hypercalcemia was linked to increased bone modeling and remodeling during the growing period [10]. However, since 2018, three cases of severe hypercalcemia needing hospitalization (albumin-corrected calcium 3.10 to 3.59 mmol/l) 4–5 months after the last denosumab injection have been published in adults. A 40-year-old man was treated with denosumab 120 mg monthly for 4 years for a giant cell tumor of the bone [7], and two post-menopausal women were treated with an aromatase inhibitor and denosumab 120 mg quarterly for 5 years [12, 13]. Extensive clinical assessment ruled out any other pathology explaining the hypercalcemia. The giant cell tumor of the bone was in remission [7], and in the other cases, hypercalcemia persisted despite correction of the newly diagnosed hyperthyroidism and antiaromatase discontinuation [13]. In all three cases, BTMs were markedly elevated at the time of hypercalcemia. In one case, bone scintigraphy performed in the oncological setting showed very high whole body bone uptake [12], supporting the hypothesis of excessive bone remodeling at denosumab discontinuation as the cause of hypercalcemia. In these three cases, high doses of denosumab may explain the development of hypercalcemia.
To date, two cases of hypercalcemia have been reported after discontinuation of denosumab 60 mg every 6 months given for osteoporosis [4, 14] without clinical consequences. A 77-year-old woman with multiple risk factors for osteoporosis, including decreased renal function, was hospitalized 6 months after the last denosumab injection due to dehydration secondary to diarrhea [14]. She had hypercalcemia and suppressed PTH and was treated by rehydration; malignancy was ruled out. A 67-year-old woman treated with denosumab for 10 years developed asymptomatic hypercalcemia with suppressed PTH 6 months after the last injection [4]. Bisphosphonates were given after the diagnosis of thoracic SVFs, and serum calcium progressively decreased. Secondary causes of osteoporosis were excluded. The authors hypothesized that hypercalcemia developed due to the long duration of denosumab treatment [4].
Hypercalcemia does not seem to be frequent at denosumab discontinuation, and was excluded in some clinical trials and case series [1, 2]. Calcemia values at denosumab discontinuation are not mentioned in the other published case series nor clinical trials. Hypercalcemia could be transiently present at the onset of the rebound effect in people without other risk factors for hypercalcemia, and may not have been observed, if the serum calcium levels were measured after this transitional period. The described patients may have experienced severe hypercalcemia for reasons related either to the patient or their pathology (baseline remodeling) or to the treatment (denosumab dosage or duration), with some patients accumulating several characteristics. Our patient was treated with denosumab 60 mg twice yearly for osteoporosis for 3 years, so neither denosumab dosage nor treatment duration can be at the origin of the acute aggravation of the hypercalcemia. The calcemia increased despite lower PTH under cinacalcet, in the context of a dramatic increase in bone remodeling, suggesting it was secondary to denosumab discontinuation.
Few data have been published on the use of denosumab in primary hyperparathyroidism. Administration of denosumab induces a transient increase in PTH values secondary to the serum calcium decrease in post-menopausal osteoporotic women with neither renal dysfunction nor primary hyperparathyroidism [18]. Prolongation of denosumab treatment in patients with primary hyperparathyroidism, which implies repeated transient increases in PTH levels, does not seem to have a stimulating effect on the PTH levels [19]. Very recently, a randomized double-blind trial compared denosumab, denosumab plus cinacalcet, or placebo, given for 1 year, in 45 patients with primary hyperparathyroidism [20]. Compared with the placebo group, BMD improved in both groups receiving denosumab, despite an increase in PTH that was not controlled by cinacalcet. At the end of the trial, patients were followed for only 4 weeks, so the consequences of discontinuing denosumab were not assessed.
Conclusions
Hypercalcemia following denosumab discontinuation has been well described in patients receiving high (oncological) or frequent doses; this has been mainly shown in children, but also in adults, as well as in patients treated with osteoporosis doses. While severe hypercalcemia is probably uncommon, in patients with pre-existent hypercalcemia or with an underlying pathology associated to high bone remodeling, severe hypercalcemia may develop with variable delay after the last denosumab injection. Systematic monitoring of serum calcium levels, i.e., before each denosumab injection, may be suggested in the follow-up of at-risk patients. Although denosumab seems to have a beneficial effect on BMD in patients with primary hyperparathyroidism, the risk of hypercalcemia upon its discontinuation should be taken into account before its introduction. In any case, denosumab should not be interrupted without further therapy.
Data availability
All data is included in the manuscript.
References
Bone, HG, Bolognese, MA, Yuen, CK, Kendler, DL, Miller, PD, Yang, YC, Grazette, L, San Martin, J, and Gallagher, JC Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 2011;96(4):972–980. https://doi.org/10.1210/jc.2010-1502
Popp AW, Zysset PK, Lippuner K (2016) Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporos Int 27(5):1917–1921. https://doi.org/10.1007/s00198-015-3458-6
Hoyer-Kuhn H, Franklin J, Allo G, Kron M, Netzer C, Eysel P, Hero B, Schoenau E, Semler O (2016) Safety and efficacy of denosumab in children with osteogenesis imperfect--a first prospective trial. J Musculoskelet Neuronal Interact 16(1):24–32
Koldkjaer Solling AS, Harslof T, Kaal A, Rejnmark L, Langdahl B (2016) Hypercalcemia after discontinuation of long-term denosumab treatment. Osteoporos Int 27(7):2383–2386. https://doi.org/10.1007/s00198-016-3535-5
Gossai N, Hilgers MV, Polgreen LE, Greengard EG (2015) Critical hypercalcemia following discontinuation of denosumab therapy for metastatic giant cell tumor of bone. Pediatr Blood Cancer 62(6):1078–1080. https://doi.org/10.1002/pbc.25393
Setsu N, Kobayashi E, Asano N, Yasui N, Kawamoto H, Kawai A, Horiuchi K (2016) Severe hypercalcemia following denosumab treatment in a juvenile patient. J Bone Miner Metab 34(1):118–122. https://doi.org/10.1007/s00774-015-0677-z
Uday S, Gaston CL, Rogers L, Parry M, Joffe J, Pearson J, Sutton D, Grimer R, Hogler W (2018) Osteonecrosis of the jaw and rebound hypercalcemia in young people treated with denosumab for giant cell tumor of bone. J Clin Endocrinol Metab 103(2):596–603. https://doi.org/10.1210/jc.2017-02025
Boyce AM, Chong WH, Yao J, Gafni RI, Kelly MH, Chamberlain CE, Bassim C, Cherman N, Ellsworth M, Kasa-Vubu JZ, Farley FA, Molinolo AA, Bhattacharyya N, Collins MT (2012) Denosumab treatment for fibrous dysplasia. J Bone Miner Res 27(7):1462–1470. https://doi.org/10.1002/jbmr.1603
Grasemann C, Schundeln MM, Hovel M, Schweiger B, Bergmann C, Herrmann R, Wieczorek D, Zabel B, Wieland R, Hauffa BP (2013) Effects of RANK-ligand antibody (denosumab) treatment on bone turnover markers in a girl with juvenile Paget’s disease. J Clin Endocrinol Metab 98(8):3121–3126. https://doi.org/10.1210/jc.2013-1143
Boyce AM (2017) Denosumab: an emerging therapy in pediatric bone disorders. Curr Osteoporos Rep 15(4):283–292. https://doi.org/10.1007/s11914-017-0380-1
Upfill-Brown A, Bukata S, Bernthal NM, Felsenfeld AL, Nelson SD, Singh A, Wesseling-Perry K, Eilber FC, Federman NC (2019) Use of denosumab in children with osteoclast bone dysplasias: report of three cases. JBMR Plus 3(10):e10210. https://doi.org/10.1002/jbm4.10210
Roux S, Massicotte MH, Huot Daneault A, Brazeau-Lamontagne L, Dufresne J (2019) Acute hypercalcemia and excessive bone resorption following anti-RANKL withdrawal: case report and brief literature review. Bone 120:482–486. https://doi.org/10.1016/j.bone.2018.12.012
Uchida T, Yamaguchi H, Kushima C, Yonekawa T, Nakazato M (2019) Elevated levels of circulating fibroblast growth factor 23 with hypercalcemia following discontinuation of denosumab. Endocr J. https://doi.org/10.1507/endocrj.EJ19-0198
Tjelum L, Eiken P (2018) Multiple vertebral fractures after denosumab discontinuation. Ugeskr Laeger 180(45) https://www.ncbi.nlm.nih.gov/pubmed/30404716
Trejo P, Rauch F, Ward L (2018) Hypercalcemia and hypercalciuria during denosumab treatment in children with osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact 18(1):76–80
Durr HR, Grahneis F, Baur-Melnyk A, Knosel T, Birkenmaier C, Jansson V, Klein A (2019) Aneurysmal bone cyst: results of an off label treatment with denosumab. BMC Musculoskelet Disord 20(1):456. https://doi.org/10.1186/s12891-019-2855-y
Chawla S, Blay JY, Rutkowski P, Le Cesne A, Reichardt P, Gelderblom H, Grimer RJ, Choy E, Skubitz K, Seeger L, Schuetze SM, Henshaw R, Dai T, Jandial D, Palmerini E (2019) Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. Lancet Oncol 20(12):1719–1729. https://doi.org/10.1016/s1470-2045(19)30663-1
Mazokopakis EE (2018) Denosumab-induced normocalcemic hyperparathyroidism in a woman with postmenopausal osteoporosis and normal renal function. Curr Drug Saf 13(3):214–216. https://doi.org/10.2174/1574886313666180608080355
Eller-Vainicher C, Palmieri S, Cairoli E, Goggi G, Scillitani A, Arosio M, Falchetti A, Chiodini I (2018) Protective effect of denosumab on bone in older women with primary hyperparathyroidism. J Am Geriatr Soc 66(3):518–524. https://doi.org/10.1111/jgs.15250
Leere JS, Karmisholt J, Robaczyk M, Lykkeboe S, Handberg A, Steinkohl E, Brondum Frokjaer J, Vestergaard P (2020) Denosumab and cinacalcet for primary hyperparathyroidism (DENOCINA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 8(5):407–417. https://doi.org/10.1016/S2213-8587(20)30063-2
Funding
Open access funding provided by University of Lausanne.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethics approval
The study was in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Camponovo, C., Aubry-Rozier, B., Lamy, O. et al. Hypercalcemia upon denosumab withdrawal in primary hyperparathyroidism: a case report and literature review. Osteoporos Int 31, 2485–2491 (2020). https://doi.org/10.1007/s00198-020-05676-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05676-7