Abstract
Summary
The aim of this study was to evaluate the association of levels of urinary total polyphenols considered as a proxy measure of polyphenol intake, with longitudinal changes of bone properties, in the InCHIANTI study. Dietary intake of polyphenols appears to be associated with future accelerated deterioration of bone health.
Introduction
Polyphenols, micronutrients ingested through plant-based foods, have antioxidant and anti-inflammatory properties and may contribute to osteoporosis prevention. We evaluated associations of high levels of urinary total polyphenols (UTP), a proxy measure of polyphenol intake, with longitudinal changes of bone properties in a representative cohort of free-living participants of the InCHIANTI study.
Methods
The InCHIANTI study enrolled representative samples from the registry list of two towns in Tuscany, Italy. Baseline data were collected in 1998 and follow-up visits in 2001 and 2004. Of the 1453 participants enrolled, 956 consented to donate a 24-h urine sample used to assess UTP, had dietary assessment, a physical examination, and underwent a quantitative computerized tomography (pQCT) of the tibia. From pQCT images, we estimated markers of bone mass (BM), diaphyseal design (DD), and material quality (MQ). Mixed models were used to study the relationship between baseline tertiles of UTP with changes of the bone characteristics over the follow-up.
Results
At baseline, higher levels of UTP were positively correlated with markers of BM, DD, and MQ. Compared with lower tertile of UTP, participants in the intermediate and highest tertiles had higher cortical bone area, cortical mineral content, and cortical thickness. However, participants in the intermediate and highest UTP tertiles experienced accelerated deterioration of these same parameters over the follow-up compared with those in the lowest UTP tertile.
Conclusions
Dietary intake of polyphenols estimated by UTP and dietary questionnaire was associated with long-term accelerated deterioration of bone health. Our study does not support the recommendation of increasing polyphenol intake for osteoporosis prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the population aging and the resulting increased prevalence of chronic diseases, the identification of life style strategies that may effectively prevent age-associated rise in chronic morbidity has become a very important theme of medical research. Prevention is the only intervention with the potential to offset the personal, social, and economic burden of age-related chronic diseases such as osteoporosis [1, 2]. Studies have suggested that specific dietary patterns may reduce the risk of osteoporosis and its harmful consequence as fractures, disability, and poor quality of life, especially if started at young age and maintained over the entire life course [3]. Indeed, epidemiological studies have shown that a large and diverse consumption of fruit could positively influence the structural and qualitative properties of the bone [4, 5]. For example, in a population of about 4000 Chinese individuals, a greater consumption of fruit was associated with lower risk of hip fracture [6].
The protective effect of fruit consumption against osteoporosis has been attributed to different compounds with polyphenols being one of the strongest candidates [5]. Polyphenols are a family of natural organic molecules, widely present in plant-derived foods. They are characterized, as the name indicates, by the presence of one or more phenolic groups, organized in more or less complex structures, generally of high molecular weight [7].
The assessment of polyphenol plasma concentration is difficult because their blood concentration is in the micromolar range. Moreover, pharmacokinetics profiles of the different classes of polyphenols, and interindividual differences in the intestinal absorption, kinetics, metabolism, and excretion could interfere with the assessment of polyphenol exposure. Urinary excretion of polyphenol metabolites is considered a good biomarker of polyphenol exposure, only minimally affected by renal function, which may be reduced in older subjects [8, 9].
The biological activity of polyphenols has been attributed to their antioxidant and anti-inflammatory properties [10, 11]. Pre-clinical studies suggested that polyphenols suppress bone resorption, either by inhibition of osteoclastogenesis and/or increased apoptosis of osteoclasts [12,13,14]. There is also some evidence that polyphenols may stimulate osteoblast maturation and survival [15]. These effects cause an increase in bone mineral density (BMD), both of the long bones and the vertebrae, as well as the modulation of the microstructural architecture of the bone [13]. In spite of these pre-clinical findings, whether dietary intake of polyphenols has beneficial effect on bone health in humans remains uncertain.
The aim of this study was to evaluate the association between urinary total polyphenols (UTP), used as a proxy measure of polyphenol intake, and longitudinal changes of bone mineralization and architecture in a cohort of free-living subjects, representative of the Italian population.
Methods
The design of the InCHIANTI study has been described in details elsewhere [16]. Briefly, the study was designed by the Laboratory of Clinical Epidemiology of the Italian National Institute of Research and Care on Aging (INRCA, Florence, Italy) and performed in two small towns in Tuscany. The baseline data were collected in 1998–2000; the 3-year follow-up took place in 2001–2003 and the 6-year follow-up in 2004–2006.
Samples
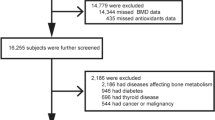
Of the 1453 participants enrolled at baseline in the InCHIANTI Study, 956 participants were included in this study because they consented to donate a urine sample and all variables of interest for this study were available. Participants were all European subjects and of Caucasian race. The Ethical Committee of the Local Health Authority of Florence, Tuscany Region, approved the study protocol, and written informed consent was obtained from each participant.
Dietary assessment
At baseline as in the subsequent follow-up, usual food consumption and energy intake were estimated through personal interviews and by the Italian version of the European Prospective Study into Cancer and Nutrition study (EPIC) [17], a tool previously validated in the Italian population [18]. The information derived from the questionnaire was converted into data on energy, micronutrient intake, and macronutrient intake, by software specifically customized for the EPIC study. For the purpose of this study, the following variables were used: total kilocalories consumed (kcal/day), total protein consumed (g/day), mean number of glasses of wine consumed/day, and total dietary polyphenols consumed (mg/day) (TDP) that were calculated according to Zamora-Ros R: TDP intake was calculated as the sum of flavonoids (anthocyanidins, flavonols, flavanones, flavones, flavanols, and isoflavones), phenolic acids, lignans, stilbenes, and other polyphenols expressed as aglycone equivalents (mg/day) [19].
Urinary total polyphenols
At baseline, 24-h urine samples were obtained from participants. Urine samples were immediately aliquoted and stored at − 80 °C until analysis. Samples were thawed on ice and analyzed using the Folin-Ciocalteau (F-C) assay after a solid-phase extraction, which allows the elimination of interfering substances that could react with the F-C assay, as described previously [20]. UTP concentrations were expressed as mg of gallic acid equivalents (GAE) per 24-h urine. UTP were recoded according to tertiles of distribution (inter-tertile ranges: 126.6–177.6 mg gallic acid eq/day).
Tibial pQCT
The peripheral quantitative computed tomography (pQCT) was performed by the XCT 2000 device (Stratec Medizintechnik, Pforzheim, Germany). A detailed description of the tibial QCT examination, in the InCHIANTI study, has been published elsewhere [21].
The images obtained from the pQCT were analyzed using the BonAlyse software (BonAlyse Oy, Jyvaskyla, Finland).
The following bone parameters were derived from the pQCT images measured at 38% tibia length [22]:
Bone mass
Total bone area (TBA): tibia cross-section total bone area, in cm2; cortical bone area (CBA): area of the cortical bone region of the tibia cross-section, in cm2; mineral content of total bone (MCBtotal): total estimated bone content in the whole tibia cross-section, in g/cm; mineral content of cortical bone (MCBcortical): amount of mineral present in the defined cortical bone area of the tibia cross section, in g/cm;
Diaphyseal design
Cortical thickness (CTh): average thickness of the circular crown formed by the centered periosteal and endocortical circumferences, in mm; periosteal perimeter (PP): external perimeter of the tibia cross section, assimilated to a circular figure (assessed automatically), in mm; medullary area (MedA): is the difference between total and cortical bone areas. It includes the marrow space and areas of the inner cortex trabecularized by endocortical resorption that have a cortical apparent vBMD < 710 mg/cm3, and is sensitive to endocortical resorption, in cm2; cross-sectional moment of inertia (CSMI): (average of maximum and minimum moment of inertia; kg/cm) is calculated as a density weighted moment of inertia. It provides a valid estimate of resistance to bending;
Material quality
Total volumetric mineral density (vBMDtot) assessed as the average density of the total bone area, in mg/cm3; cortical volumetric mineral density (vBMDc) a selective measure of the apparent volumetric density of cortical bone, in mg/cm3;
Lastly, using pQCT images, we also estimated calf muscle cross-sectional area (CMCSA) from a transverse scan performed at 66% of the tibia length from the distal tip of the tibia, which is the level of largest outer calf diameters, and expressed in cm2.
Covariate
Laboratory assay
Fasting blood samples were collected in the morning after a 12-h fast, centrifuged, and stored at − 80 °C. Serum levels of vitamin D (25OHD, nmol/L) were measured by radioimmunoassay (RIA kit; DiaSorin, Stillwater, MN). The intraassay and interassay coefficients of variation for vitamin D were 8.1% and 10.2%, respectively. Serum intact parathyroid hormone (PTH, pg/mL) levels were measured with a two-site immunoradiometric assay kit (N-tact PTHSP; DiaSorin). The intraassay and interassay coefficients of variation for PTH were < 3.0% and 5.5%, respectively.
Total plasma proteins (g/dL) and serum creatinine level (mg/dL) were measured by the Laboratory of Clinical Chemistry and Microbiological Assays, SS. Annunziata Hospital, Azienda Sanitaria 10, Florence, Italy, using a colorimetric assay (TP, Roche Diagnostics, GmbH, Mannheim, Germany) and a Roche analyzer (Roche Diagnostics, GmbH, Mannheim, Germany). At baseline, the analyzer was a Hitachi 917. For the follow-ups, it was a Modular P800 Hitachi.
Glomerular filtration rate was calculated according to Cockcroft-Gault formula [23].
Physical performance and strength
The short physical performance battery (SPPB) based on the lower-extremity performance tests used in the Established Populations for the Epidemiologic Studies of the Elderly (EPESE) was used here to summarize lower extremity performance [24]. The SPPB consisted of walking speed, ability to stand from a chair, and ability to maintain balance in progressively more challenging positions. Each physical performance measure was categorized into a five-level score, with 0 representing inability to do the test and 4 representing the highest level of performance. The three measures were then added to create a summary physical performance measure ranging from 0 (worst) to 12 (best).
Handgrip strength was measured using a handheld dynamometer (hydraulic hand “BASELINE”; Smith & Nephew, Agrate Brianza, Milan, Italy). Participants were asked to perform the task twice with each hand. The average of the best result obtained with each hand was used for these analyses.
Information on smoking status was collected at home interview. BMI (in kg/m2) was calculated using measured weight (in kg) divided by height (in m2).
Statistical analysis
Baseline characteristics were compared between tertiles of distribution of UTP, for all the variables of interest; differences among tertiles were evaluated using analysis of variance for continuous variables and χ2 test analyses for dichotomous or categorical variables; moreover, in the descriptive table, the p values adjusted for age, sex, and GFR were also reported using, respectively, linear and logistic regression models.
Differences in the bone health markers between groups, at baseline and at follow-up, were tested by mixed model analysis of covariance. The advantage of this statistical approach is improvement in the precision of the effect estimate by using all available information and, at the same time, allowing for the handling of missing data [25].
Model A reported the unconditional means model, which evaluated just the random effect for the intercept without any predictors; model B reported the unconditional growth model, which considers the effect of time; model C also includes an interaction term between time and UTP tertiles (the classification variable); model D was the fully adjusted model, considering all the potential confounders: age, sex, handgrip test, and SPPB score, smoking status, serum protein level, and variation across time of creatinine clearance.
Akaike’s information criterion (AIC) and Bayesian information criterion (BIC) were used to examine improvement in model fit, for both of these measurements, smaller values represent better fitting models. To estimate a possible interference of regression to the mean (RTM) in our findings, in a sensitivity analysis, we performed ANCOVA models adjusted for baseline values of the bone parameters [26]. Since the results of these models were substantially consistent with those obtained using the simple regression approach, they are not reported in the results section.
Results
Cross-sectional analysis
Body composition and laboratory parameters according to tertiles of UTP are shown in Table 1. P values for unadjusted and adjusted (for age, sex, and GFR) comparisons by UTP tertiles are shown, respectively, in columns A and B.
In the fully adjusted model, SPPB and handgrip were significantly higher according to higher tertiles of UTP. As expected, DTP was higher in those subjects who were classified in the highest tertile of UTP (p < 0.001).
In Table 2, average baseline pQCT parameters, namely diaphyseal architecture, mass, and bone quality, are reported according to UTP tertiles. Bone mass parameters, specifically the TBA and the CBA at 38% of the tibial length, were significantly higher, in the highest tertile of UTP. On the contrary, the MedA was smaller in the highest tertile compared with the other two tertiles. Adjusting the analysis for age, sex, and GFR, the differences in MedA were no longer statistically significant (p = 0.08). Diaphyseal architectural characteristics, specifically the CTh and the CSMI, were significantly higher in the high tertile of UTP compared with the intermediate and lowest tertiles. Finally, bone material characteristics, namely vBMDcortical and total vBMDtotal, were both significantly higher in the highest tertile of UTP compared with the two lower tertiles.
Longitudinal analysis
Among markers of bone health, only CBA, CMC, and CTh showed different rates of change over time according to tertiles of UTP. Differences in baseline UTP among participants explained 57%, 43%, and 26% of the total variation of, respectively, CBA, CMC, and CTh (Tables 3, 4, and 5, mixed models A: unconditional means model). When the effect of time was considered (model B, unconditional growth model), for BCA and CTh, 62% of the variance was explained by the linear association of UTP variability with time, whereas for CMC, the percentage rose to 80%.
The rate of change in every single time of the study (γ10) was − 0.315 ± 0.009 (p < 0.001) for BCA, − 0.116 ± 0.002 (p < 0.001) for CMC, and − 0.691 ± 0.019 (p < 0.001) for CTh.
In the analysis for the BCA, CMC as for the CTh, since covariance (δ01) is statistically significant, it could be argued that there is a relationship between the starting level and the rate of change; in other words, the rate of change during time is greater in the higher UTP tertile.
Moreover, for the three markers, tertiles of UTP could explain: 7.2 and 7.3% of the differences among the three groups at the baseline (R20) for BCA and CTh, whereas 8.5% for CMC, respectively, 11.1% and 13.8% and 14.3% in the variation in the rate of change (R21).
Compared with lower tertile of UTP, the higher and the intermediate tertiles, at baseline, had a greater BCA, CMC, and CTh (γ01 I-H); moreover, during the follow-up, those two tertiles of UTP lose a statistically significant higher BCA, CMC, and CTh (γ11 I-H).
In the fully adjusted models (model D), the results did not substantially change; the percentage of the explained increase in the differences among the three groups at baseline (respectively for BCA = 65.2%, CMC = 53.5%, and for CTh = 31.8%), and in the variation in the rate of change (respectively, for BCA = 33.3%, CMC = 14.5%, and for CTh14.7%). The fit statistic (AIC and BIC) improved for more complex models in all the bone markers considered.
To assess whether a “regression to the mean” could account for all or part of our findings, we ran an ANCOVA model adjusted for baseline level of the bone parameter of interest, and the results were substantially unchanged (cortical bone area p = 0.006; cortical mineral content p < 0.001; cortical thickness p = 0.04).
Discussion
In this study, we found that those individuals with higher baseline urinary levels of polyphenols had larger BCA, CMC, and CTh. However, during the 6-year follow-up, participants in the highest and intermediate compared with lowest tertile of UTP showed accelerated deterioration of the same parameters. Baseline urinary levels of polyphenols were also cross-sectionally associated with parameters of bone mass, diaphyseal design, and material quality, but baseline polyphenol levels not, with differential changes over time in the same parameters.
There is ample evidence that lifestyles and nutrition may affect bone quality [6, 12, 27]. In particular, it has been suggested that polyphenols, active compounds present in many fruits and vegetable may have beneficial effect in chronic disease prevention, and in particular osteoporosis [27].
Polyphenols may affect bone remodeling through the inhibition, differentiation, and activation of the apoptotic processes of osteoclasts [12]. On the other hand, there is also some evidence that they promote the expansion of the osteoblast line through the activation of bone morphogenic protein (BMP) [15]. Lastly, polyphenols are anti-inflammatory, although the specific mechanism that mediate this activity is not completely understood [13]. Through these mechanisms, dietary intake of polyphenols may improve BMD and reduce its rate of decline with aging, therefore reducing the risk of fractures. Indeed, supplementation of foods enriched with polyphenols prevents the reduction in cortical thickness and the medullary area in mice [28, 29]. Also, the microscopic structure could be influenced in a dose-dependent manner by supplementation with foods enriched in polyphenols, in detail the porosity of the cortex could be influenced with larger fibers and smaller interfibrillar spaces [30].Our findings are both supportive and in contrast with this literature. UTP is a likely a biomarker of the exposure (intake) of polyphenol in the short terms and its positive association with markers of bone health (volumetric bone density, geometric properties, and diaphyseal design) independent of potential confounders is in keeping with the literature. However, the results of the longitudinal analysis are surprisingly pointing to a different direction. We found that high values of UTP were associated with accelerated bone remodeling, a process that can lead to osteoporosis.
It is difficult to speculate on the meaning of these unexpected findings. A possibility is that polyphenols form robust, even if reversible complexes with ions and in particular with iron and calcium [31]. Initially, those complexes could facilitate the growth of hydroxyapatite crystals, but later polyphenols bind calcium and facilitate its urinary excretion, therefore reducing its availability for bone building [32]. This hypothesis is consistent with the observation that higher consumption of polyphenols is associated with lower prevalence of kidney stones, and among the many hypotheses, a mechanism of action considered is the high affinity of polyphenols for the urinary ions [33, 34]. An alternative hypothesis is that the decline of GFR with aging could interfere with bone directly and could facilitate the urinary formation of complex polyphenol-calcium, reducing the bioavailability of serum calcium [19, 20].
Regardless of possible mechanistic interpretations of our findings, they suggest cautions in recommending high intake of polyphenols as a prevention strategy for osteoporosis and suggest that further studies in this research area are needed, possibly including longitudinal data and objective measures of polyphenols intake collected over time.
An important limitation of our study is the use of urinary values of polyphenols as a marker of polyphenol exposure, which may not be fully representative of blood levels and/or dietary intake.
Previous studies have found that for most polyphenols, the urinary excretion values are proportional to circulating levels but we cannot exclude that the age associated decline of GFR of the presence of other interfering molecules introduce random or a systematic error in the urine assay. On the other hand, it is likely that urinary excretion is a better measure of exposure than estimated dietary intake, both because the reliability of dietary questionnaire tends to be modest and because food matrix or background diet may affect absorption and bioavailability of polyphenols. Urine sampling is particularly useful for polyphenols with short half-lives, where plasma measurements may fail to monitor even acute intake [33, 34]. Indeed, as reviewed elsewhere, and even more, specifically for this measurement, as validated by other researchers of the INCHIANTI group, urinary assessment of polyphenols is a reliable and valid method [19, 20].
Another limitation of our study is that bone measures were performed on the tibia. It has been shown that geometric and quality properties of the cortical and trabecular bone display only moderate correlations among the different sites of the skeleton [35]. Altogether, these data suggest that density measurements of one anatomical region are not necessarily accurate measures of other regions [36]. Therefore, our data may not be fully representative of bone events that occur in the femur and vertebral bodies, which are the typical sites for the assessment of osteoporosis, and the most frequently involved in osteoporosis-related adverse events. Furthermore, UTP were evaluated only at baseline, so we do not know how the urinary concentrations changed during the study in our population, and how this may have influenced the results. Different polyphenols may have different effects, and the total may result from various admixtures in the subjects, accounting for the dual effect observed.
Lastly, one of the most important concerns when analyzing repeated measures is RTM: mixed models estimates can also be influenced by RTM. However, also taking into account this possible statistical artifact, the analysis did not change the overall meaning of the results.
Conclusions
We found that higher urinary concentrations of polyphenols were associated with positive cross-sectional effects on biomarkers of bone health, but were associated with a subsequent accelerated worsening of the same biomarkers. These contradictory results should be further evaluated in longitudinal studies including multiple measures of polyphenol exposure and bone health. In the light of our findings, the use of dietary polyphenols for osteoporosis prevention should be considered with caution.
Data availability
The InCHIANTI dataset is not deposit in a data repository, but data could be available on request.
References
Fabiani R, Naldini G, Chiavarini M (2019) Dietary patterns and metabolic syndrome in adult subjects: a systematic review and meta-analysis. Nutrients 11:2056. https://doi.org/10.3390/nu11092056
Rizzoli R, Biver E, Bonjour J-P, Coxam V, Goltzman D, Kanis JA, Lappe J, Rejnmark L, Sahni S, Weaver C, Weiler H, Reginster JY (2018) Benefits and safety of dietary protein for bone health. Osteoporos Int 29:1933–1948. https://doi.org/10.1007/s00198-018-4534-5
Pedone C, Napoli N, Pozzilli P et al (2011) Dietary pattern and bone density changes in elderly women: a longitudinal study. J Am Coll Nutr. https://doi.org/10.1080/07315724.2011.10719954
Byberg L, Bellavia A, Larsson SC, Orsini N, Wolk A, Michaëlsson K (2016) Mediterranean diet and hip fracture in Swedish men and women. J Bone Miner Res 31:2098–2105. https://doi.org/10.1002/jbmr.2896
Arjmandi BH, Johnson SA, Pourafshar S, Navaei N, George KS, Hooshmand S, Chai SC, Akhavan NS (2017) Bone-protective effects of dried plum in postmenopausal women: efficacy and possible mechanisms. Nutrients 9. https://doi.org/10.3390/nu9050496
Muraki S, Yamamoto S, Ishibashi H, Oka H, Yoshimura N, Kawaguchi H, Nakamura K (2007) Diet and lifestyle associated with increased bone mineral density: cross-sectional study of Japanese elderly women at an osteoporosis outpatient clinic. J Orthop Sci 12:317–320. https://doi.org/10.1007/s00776-007-1143-0
Blanton C (2018) Bone response to dietary co-enrichment with powdered whole grape and probiotics. Nutrients. 10. https://doi.org/10.3390/nu10020146
Spencer JPE, Abd El Mohsen MM, Minihane AM, Mathers JC (2008) Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr 99:12–22. https://doi.org/10.1017/S0007114507798938
Rothwell JA, Knaze V, Zamora-Ros R (2017) Polyphenols: dietary assessment and role in the prevention of cancers. Curr Opin Clin Nutr Metab Care 20:512–521. https://doi.org/10.1097/MCO.0000000000000424
Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L (2005) Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 45:287–306. https://doi.org/10.1080/1040869059096
Li X, Lin J, Chen B, Xie H, Chen D (2018) Antioxidant and cytoprotective effects of kukoamines A and B: comparison and positional isomeric effect. Molecules. 23. https://doi.org/10.3390/molecules23040973
Shen CL, Smith BJ, Li J, Cao JJ, Song X, Newhardt MF, Corry KA, Tomison MD, Tang L, Wang JS, Chyu MC (2019) Effect of long-term green tea polyphenol supplementation on bone architecture, turnover, and mechanical properties in middle-aged ovariectomized rats. Calcif Tissue Int 104:285–300. https://doi.org/10.1007/s00223-018-0489-y
Torre E (2017) Molecular signaling mechanisms behind polyphenol-induced bone anabolism. Phytochem Rev 16:1183–1226. https://doi.org/10.1007/s11101-017-9529-x
Domazetovic V (2017) Oxidative stress in bone remodeling: role of antioxidants. Clin Cases Miner Bone Metab 14:209–216. https://doi.org/10.11138/ccmbm/2017.14.1.209
Shahnazari M, Turner RT, Iwaniec UT, Wronski TJ, Li M, Ferruzzi MG, Nissenson RA, Halloran BP (2016) Dietary dried plum increases bone mass, suppresses proinflammatory cytokines and promotes attainment of peak bone mass in male mice. J Nutr Biochem 34:73–82. https://doi.org/10.1016/j.jnutbio.2016.04.007
Ferrucci L, Bandinelli S, Benvenuti E, di Iorio A, Macchi C, Harris TB, Guralnik JM (2000) Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc 48:1618–1625. https://doi.org/10.1111/j.1532-5415.2000.tb03873.x
Pisani P (1997) Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol 26:152S–1160S. https://doi.org/10.1093/ije/26.suppl_1.s152
Bartali B, Turrini A, Salvini S, Lauretani F, Russo CR, Corsi AM, Bandinelli S, D’Amicis A, Palli D, Guralnik JM, Ferrucci L (2004) Dietary intake estimated using different methods in two Italian older populations. Arch Gerontol Geriatr 38:51–60. https://doi.org/10.1016/S0167-4943(03)00084-0
Zamora-Ros R, Achaintre D, Rothwell JA, Rinaldi S, Assi N, Ferrari P, Leitzmann M, Boutron-Ruault MC, Fagherazzi G, Auffret A, Kühn T, Katzke V, Boeing H, Trichopoulou A, Naska A, Vasilopoulou E, Palli D, Grioni S, Mattiello A, Tumino R, Ricceri F, Slimani N, Romieu I, Scalbert A (2016) Urinary excretions of 34 dietary polyphenols and their associations with lifestyle factors in the EPIC cohort study. Sci Rep 6. https://doi.org/10.1038/srep26905
Zamora-Ros R, Rabassa M, Cherubini A, Urpi-Sarda M, Llorach R, Bandinelli S, Ferrucci L, Andres-Lacueva C (2011) Comparison of 24-h volume and creatinine-corrected total urinary polyphenol as a biomarker of total dietary polyphenols in the Invecchiare InCHIANTI study. Anal Chim Acta 704:110–115. https://doi.org/10.1016/j.aca.2011.07.035
Russo CR, Lauretani F, Bandinelli S, Bartali B, di Iorio A, Volpato S, Guralnik JM, Harris T, Ferrucci L (2003) Aging bone in men and women: beyond changes in bone mineral density. Osteoporos Int 14:531–538. https://doi.org/10.1007/s00198-002-1322-y
Capozza RF, Feldman S, Mortarino P, Reina PS, Schiessl H, Rittweger J, Ferretti JL, Cointry GR (2010) Structural analysis of the human tibia by tomographic (pQCT) serial scans. J Anat 216:470–481. https://doi.org/10.1111/j.1469-7580.2009.01201.x
Garasto S, Fusco S, Corica F, Rosignuolo M, Marino A, Montesanto A, de Rango F, Maggio M, Mari V, Corsonello A, Lattanzio F (2014) Estimating glomerular filtration rate in older people. Biomed Res Int 2014:1–12. https://doi.org/10.1155/2014/916542
Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB (1995) Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 332:556–562. https://doi.org/10.1056/NEJM199503023320902
Littell RC, Pendergast J, Natarajan R (2000) Modelling covariance structure in the analysis of repeated measures data. Stat Med 19:1793–1819. https://doi.org/10.1002/1097-0258(20000715)19:13<1793::AID-SIM482>3.0.CO;2-Q|
Barnett AG, van der Pols JC, Dobson AJ (2005) Regression to the mean: what it is and how to deal with it. Int J Epidemiol 34:215–220. https://doi.org/10.1093/ije/dyh299
Qiu R, Cao WT, Tian HY, He J, Chen GD, Chen YM (2017) Greater intake of fruit and vegetables is associated with greater bone mineral density and lower osteoporosis risk in middle-aged and elderly adults. PLoS One 12:e0168906. https://doi.org/10.1371/journal.pone.0168906
Ejima H, Richardson JJ, Liang K et al (2013) One-step assembly of coordination complexes for versatile film and particle engineering. Science (80- ). https://doi.org/10.1126/science.1237265
Holten-Andersen N, Fantner GE, Hohlbauch S, Waite JH, Zok FW (2007) Protective coatings on extensible biofibres. Nat Mater 6:669–672. https://doi.org/10.1038/nmat1956
Prajatelistia E, Ju S-W, Sanandiya ND, Jun SH, Ahn JS, Hwang DS (2016) Tunicate-inspired gallic acid/metal ion complex for instant and efficient treatment of dentin hypersensitivity. Adv Healthc Mater 5:919–927. https://doi.org/10.1002/adhm.201500878
Ahmed S, Hasan MM, Khan H, Mahmood ZA, Patel S (2018) The mechanistic insight of polyphenols in calcium oxalate urolithiasis mitigation. Biomed Pharmacother 106:1292–1299. https://doi.org/10.1016/j.biopha.2018.07.080
Kurajoh M, Inaba M, Nagata Y, Yamada S, Imanishi Y, Emoto M (2019) Association of cystatin C- and creatinine-based eGFR with osteoporotic fracture in Japanese postmenopausal women with osteoporosis: sarcopenia as risk for fracture. J Bone Miner Metab 37:282–291. https://doi.org/10.1007/s00774-018-0913-4
Manach C, Williamson G, Morand C, Scalbert A, Rémésy C (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81:230S–242S. https://doi.org/10.1093/ajcn/81.1.230S
Williamson G, Manach C (2005) Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 81:243S–255S. https://doi.org/10.1093/ajcn/81.1.243S
Lochmüller E-M, Groll O, Kuhn V, Eckstein F (2002) Mechanical strength of the proximal femur as predicted from geometric and densitometric bone properties at the lower limb versus the distal radius. Bone 30:207–216. https://doi.org/10.1016/S8756-3282(01)00621-4
Chirchir H (2016) Limited trabecular bone density heterogeneity in the human skeleton. Anat Res Int 2016:1–7. https://doi.org/10.1155/2016/9295383
Funding
Open access funding provided by Università degli Studi G. D'Annunzio Chieti Pescara within the CRUI-CARE Agreement. The InCHIANTI study was supported as a “targeted project” (ICS 110.1/RS97.71) by the Italian Ministry of Health and by the U.S. National Institute on Aging (contracts N01-AG-916413, N01-AG-5-0002, and N01-AG-821336, and grant R01-AG-027012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Di Iorio, A., Abate, M., Bandinelli, S. et al. Total urinary polyphenols and longitudinal changes of bone properties. The InCHIANTI study. Osteoporos Int 32, 353–362 (2021). https://doi.org/10.1007/s00198-020-05585-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05585-9