Abstract
Summary
Estrogen receptor (ER) in ovariectomy-induced osteoporotic fracture was reported to exhibit delayed expression. Mechanical stimulation enhanced ER-α expression in osteoporotic fracture callus at the tissue level. ER was also found to be required for the effectiveness of vibrational mechanical stimulation treatment in osteoporotic fracture healing.
Introduction
Estrogen receptor(ER) is involved in mechanical signal transduction in bone metabolism. Its expression was reported to be delayed in osteoporotic fracture healing. The purpose of this study was to investigate the roles played by ER during osteoporotic fracture healing enhanced with mechanical stimulation.
Methods
Ovariectomy-induced osteoporotic SD rats that received closed femoral fractures were divided into five groups, (i) SHAM, (ii) SHAM-VT, (iii) OVX, (iv) OVX-VT, and (v) OVX-VT-ICI, where VT stands for whole-body vibration treatment and ICI for ER antagonization by ICI 182,780. Callus formation and gene expression were assessed at 2, 4, and 8 weeks postfracture. In vitro osteoblastic differentiation, mineralization, and ER-α expression were assessed.
Results
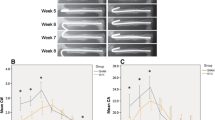
The delayed ER expression was found to be enhanced by vibration treatment. Callus formation enhancement was shown by callus morphometry and micro-CT analysis. Enhancement effects by vibration were partially abolished when ER was modulated by ICI 182,780, in terms of callus formation capacity at 2–4 weeks and ER gene and protein expression at all time points. In vitro, ER expression in osteoblasts was not enhanced by VT treatment, but osteoblastic differentiation and mineralization were enhanced under estrogen-deprived condition. When osteoblastic cells were modulated by ICI 182,780, enhancement effects of VT were eliminated.
Conclusions
Vibration was able to enhance ER expression in ovariectomy-induced osteoporotic fracture healing. ER was essential in mechanical signal transduction and enhancement in callus formation effects during osteoporotic fracture healing enhanced by vibration. The enhancement of ER-α expression by mechanical stimulation was not likely to be related to the increased expression in osteoblastic cells but rather to the systemic enhancement in recruitment of ER-expressing progenitor cells through increased blood flow and neo-angiogenesis. This finding might explain the observed difference in mechanical sensitivity of osteoporotic fracture to mechanical stimulation.





Similar content being viewed by others
References
Akesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, Kyer C, Cooper C (2013) Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int 24:2135–2152
Ho KS, Chan WM (2003) Falls in Elderly - A “Clinical Syndrome” and a Public Health Issue. In Department of Health HK (ed). Public Health & Epidemiology Bulletin, Hong Kong, pp 13-17
Mithal A, Ebeling P (2013) Epidemiology, costs & burden of osteoporosis in 2013. In: Jagait CK, Misteli L, Pierroz D (eds) The Asia-pacific regional audit. International Osteoporosis Foundation, IOF, Asia-Pacific
Cheung WH, Sun MH, Zheng YP, Chu WC, Leung AH, Qin L, Wei FY, Leung KS (2012) Stimulated angiogenesis for fracture healing augmented by low-magnitude, high-frequency vibration in a rat model-evaluation of pulsed-wave doppler, 3-D power Doppler ultrasonography and micro-CT microangiography. Ultrasound Med Biol 38:2120–2129
Chow DH, Leung KS, Qin L, Leung AH, Cheung WH (2011) Low-magnitude high-frequency vibration (LMHFV) enhances bone remodeling in osteoporotic rat femoral fracture healing. J Orthop Res 29:746–752
Leung KS, Shi HF, Cheung WH, Qin L, Ng WK, Tam KF, Tang N (2009) Low-magnitude high-frequency vibration accelerates callus formation, mineralization, and fracture healing in rats. J Orthop Res 27:458–465
Shi HF, Cheung WH, Qin L, Leung AH, Leung KS (2010) Low-magnitude high-frequency vibration treatment augments fracture healing in ovariectomy-induced osteoporotic bone. Bone 46:1299–1305
Gruber CJ, Tschugguel W, Schneeberger C, Huber JC (2002) Production and actions of estrogens. N Engl J Med 346:340–352
Planey SL, Kumar R, Arnott JA (2014) Estrogen receptors (ERalpha versus ERbeta): friends or foes in human biology? J Recept Signal Transduct Res 34:1–5
Jessop HL, Suswillo RF, Rawlinson SC, Zaman G, Lee K, Das-Gupta V, Pitsillides AA, Lanyon LE (2004) Osteoblast-like cells from estrogen receptor alpha knockout mice have deficient responses to mechanical strain. J Bone Miner Res 19:938–946
Zaman G, Jessop HL, Muzylak M, De Souza RL, Pitsillides AA, Price JS, Lanyon LL (2006) Osteocytes use estrogen receptor alpha to respond to strain but their ERalpha content is regulated by estrogen. J Bone Miner Res 21:1297–1306
Moverare S, Venken K, Eriksson AL et al (2003) Differential effects on bone of estrogen receptor alpha and androgen receptor activation in orchidectomized adult male mice. Proc Natl Acad Sci U S A 100:13573–13578
Neidlinger-Wilke C, Stalla I, Claes L, Brand R, Hoellen I, Rubenacker S, Arand M, Kinzl L (1995) Human osteoblasts from younger normal and osteoporotic donors show differences in proliferation and TGF beta-release in response to cyclic strain. J Biomech 28:1411–1418
Aguirre JI, Plotkin LI, Gortazar AR, Millan MM, O’Brien CA, Manolagas SC, Bellido T (2007) A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J Biol Chem 282:25501–25508
Sunters A, Armstrong VJ, Zaman G, Kypta RM, Kawano Y, Lanyon LE, Price JS (2010) Mechano-transduction in osteoblastic cells involves strain-regulated estrogen receptor alpha-mediated control of insulin-like growth factor (IGF) I receptor sensitivity to Ambient IGF, leading to phosphatidylinositol 3-kinase/AKT-dependent Wnt/LRP5 receptor-independent activation of beta-catenin signaling. J Biol Chem 285:8743–8758
Chow SK, Leung KS, Qin L, Wei F, Cheung WH (2014) Callus formation is related to the expression ratios of estrogen receptors-alpha and -beta in ovariectomy-induced osteoporotic fracture healing. Arch Orthop Trauma Surg 134:1405–1416
Cheung WH, Chow SK, Sun MH, Qin L, Leung KS (2011) Low-intensity pulsed ultrasound accelerated callus formation, angiogenesis and callus remodeling in osteoporotic fracture healing. Ultrasound Med Biol 37:231–238
Chow DH, Leung KS, Qin L, Leung AH, Cheung WH (2010) Low-magnitude high-frequency vibration (LMHFV) enhances bone remodeling in osteoporotic rat femoral fracture healing. J Orthop Res
Chung SL, Leung KS, Cheung WH (2014) Low-magnitude high-frequency vibration enhances gene expression related to callus formation, mineralization and remodeling during osteoporotic fracture healing in rats. J Orthop Res 32:1572–1579
Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138:863–870
Sibonga JD, Dobnig H, Harden RM, Turner RT (1998) Effect of the high-affinity estrogen receptor ligand ICI 182,780 on the rat tibia. Endocrinology 139:3736–3742
Siu WS, Qin L, Cheung WH, Leung KS (2004) A study of trabecular bones in ovariectomized goats with micro-computed tomography and peripheral quantitative computed tomography. Bone 35:21–26
Li F, Chow S, Cheung WH, Chan FL, Chen S, Leung LK (2013) The citrus flavonone hesperetin prevents letrozole-induced bone loss in a mouse model of breast cancer. J Nutr Biochem 24:1112–1116
Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T (2001) Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone 28:80–86
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Bellows CG, Aubin JE, Heersche JN, Antosz ME (1986) Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int 38:143–154
Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS (1990) Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143:420–430
Lazzaro VA, Walker RJ, Duggin GG, Phippard A, Horvath JS, Tiller DJ (1992) Inhibition of fibroblast proliferation in L-valine reduced selective media. Res Commun Chem Pathol Pharmacol 75:39–48
Damien E, Price JS, Lanyon LE (1998) The estrogen receptor’s involvement in osteoblasts’ adaptive response to mechanical strain. J Bone Miner Res 13:1275–1282
Gough JE, Notingher I, Hench LL (2004) Osteoblast attachment and mineralized nodule formation on rough and smooth 45S5 bioactive glass monoliths. J Biomed Mater Res A 68:640–650
Tam KF, Cheung WH, Lee KM, Qin L, Leung KS (2005) Delayed stimulatory effect of low-intensity shockwaves on human periosteal cells. Clin Orthop Relat Res 438:260–265
Tam KF, Cheung WH, Lee KM, Qin L, Leung KS (2008) Osteogenic effects of low-intensity pulsed ultrasound, extracorporeal shockwaves and their combination—an in vitro comparative study on human periosteal cells. Ultrasound Med Biol 34:1957–1965
Masuda A, Nishikawa T, Yamamoto T, Kobayashi M (2008) Simple method for Photoshop-aided double immunohistochemistry—usage of ‘image stack’ function. Histopathology 53:609–610
Matkowskyj KA, Schonfeld D, Benya RV (2000) Quantitative immunohistochemistry by measuring cumulative signal strength using commercially available software photoshop and matlab. J Histochem Cytochem 48:303–312
Boden SD, Joyce ME, Oliver B, Heydemann A, Bolander ME (1989) Estrogen receptor mRNA expression in callus during fracture healing in the rat. Calcif Tissue Int 45:324–325
Cheung WH, Chin WC, Qin L, Leung KS (2011) Low intensity pulsed ultrasound enhances fracture healing in both ovariectomy-induced osteoporotic and age-matched normal bones. J Orthop Res
Komrakova M, Sehmisch S, Tezval M et al (2013) Identification of a vibration regime favorable for bone healing and muscle in estrogen-deficient rats. Calcif Tissue Int 92:509–520
Stuermer EK, Komrakova M, Werner C et al (2010) Musculoskeletal response to whole-body vibration during fracture healing in intact and ovariectomized rats. Calcif Tissue Int 87:168–180
Wehrle E, Liedert A, Heilmann A et al (2015) The impact of low-magnitude high-frequency vibration on fracture healing is profoundly influenced by the oestrogen status in mice. Dis Model Mech 8:93–104
Damien E, Price JS, Lanyon LE (2000) Mechanical strain stimulates osteoblast proliferation through the estrogen receptor in males as well as females. J Bone Miner Res 15:2169–2177
Galea GL, Meakin LB, Sugiyama T, Zebda N, Sunters A, Taipaleenmaki H, Stein GS, van Wijnen AJ, Lanyon LE, Price JS (2013) Estrogen receptor alpha mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor beta. J Biol Chem 288:9035–9048
Liedert A, Wagner L, Seefried L, Ebert R, Jakob F, Ignatius A (2010) Estrogen receptor and Wnt signaling interact to regulate early gene expression in response to mechanical strain in osteoblastic cells. Biochem Biophys Res Commun 394:755–759
Lima F, Vico L, Lafage-Proust MH, van der Saag P, Alexandre C, Thomas T (2004) Interactions between estrogen and mechanical strain effects on U2OS human osteosarcoma cells are not influenced by estrogen receptor type. Bone 35:1127–1135
Zhou S, Zilberman Y, Wassermann K, Bain SD, Sadovsky Y, Gazit D (2001) Estrogen modulates estrogen receptor alpha and beta expression, osteogenic activity, and apoptosis in mesenchymal stem cells (MSCs) of osteoporotic mice. J Cell Biochem Suppl 36:144–155
Wei FY, Leung KS, Li G, Qin J, Chow SK, Huang S, Sun MH, Qin L, Cheung WH (2014) Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor-1 signaling in fracture healing. PLoS One 9, e106722
Yuan L, Sakamoto N, Song G, Sato M (2013) Low-level shear stress induces human mesenchymal stem cell migration through the SDF-1/CXCR4 axis via MAPK signaling pathways. Stem Cells Dev 22:2384–2393
Kim SY, Park SH, Shin JW, Kang YG, Jeon KJ, Hyun JS, Oh MJ (2013) Mechanical stimulation and the presence of neighboring cells greatly affect migration of human mesenchymal stem cells. Biotechnol Lett 35:1817–1822
Fong EL, Chan CK, Goodman SB (2011) Stem cell homing in musculoskeletal injury. Biomaterials 32:395–409
Weitzmann MN, Pacifici R (2006) Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest 116:1186–1194
Kelly DJ, Jacobs CR (2010) The role of mechanical signals in regulating chondrogenesis and osteogenesis of mesenchymal stem cells. Birth Defects Res C Embryo Today 90:75–85
Wu QQ, Chen Q (2000) Mechanoregulation of chondrocyte proliferation, maturation, and hypertrophy: ion-channel dependent transduction of matrix deformation signals. Exp Cell Res 256:383–391
Okazaki R, Inoue D, Shibata M, Saika M, Kido S, Ooka H, Tomiyama H, Sakamoto Y, Matsumoto T (2002) Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) alpha or beta. Endocrinology 143:2349–2356
Duncan RL, Turner CH (1995) Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 57:344–358
Imai Y, Kondoh S, Kouzmenko A, Kato S (2010) Minireview: osteoprotective action of estrogens is mediated by osteoclastic estrogen receptor-alpha. Mol Endocrinol 24:877–885
Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O’Brien CA, Manolagas SC (2010) The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol 24:323–334
Rubinacci A, Marenzana M, Cavani F et al (2008) Ovariectomy sensitizes rat cortical bone to whole-body vibration. Calcif Tissue Int 82:316–326
He YX, Liu Z, Pan XH et al (2012) Deletion of estrogen receptor beta accelerates early stage of bone healing in a mouse osteotomy model. Osteoporos Int 23:377–389
Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C (2003) Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a "ying yang" relationship between ERalpha and ERbeta in mice. Mol Endocrinol 17:203–208
Liu Y, Gao H, Marstrand TT, Strom A, Valen E, Sandelin A, Gustafsson JA, Dahlman-Wright K (2008) The genome landscape of ERalpha- and ERbeta-binding DNA regions. Proc Natl Acad Sci U S A 105:2604–2609
Acknowledgments
This project was supported by the National Natural Science Foundation of China (NSFC) (Reference: A.03.15.02401), the Asian Association for Dynamic Osteosynthesis Research Fund (AADO-RF2010-001-2Y), and the Direct Grant for Research of the Chinese University of Hong Kong (2009.1.044).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Wing-Hoi Cheung, Simon Kwoon-Ho Chow, Kwok Sui Leung, Jianghui Qin, Anyun Guo, Minghui Sun, and Ling Qin declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1565 kb)
Rights and permissions
About this article
Cite this article
Chow, S.K.H., Leung, K.S., Qin, J. et al. Mechanical stimulation enhanced estrogen receptor expression and callus formation in diaphyseal long bone fracture healing in ovariectomy-induced osteoporotic rats. Osteoporos Int 27, 2989–3000 (2016). https://doi.org/10.1007/s00198-016-3619-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3619-2