Abstract
Summary
We used two methods of identifying women who reached the target for raloxifene treatment with bone turnover markers. Both approaches identified women that responded to treatment but did not fully agree and may be complementary.
Introduction
The change in bone turnover markers (BTMs) in response to osteoporosis therapy can be assessed by a decrease beyond the least significant change (LSC) or below the mean of the reference interval (RI). We compared the performance of these two approaches in women treated with raloxifene.
Methods
Fifty postmenopausal osteopenic women (age 51–72 years) were randomised to raloxifene or no treatment for 2 years. Blood samples were collected for the measurement of BTM. The LSC for each marker was calculated from the untreated women and the RI obtained from healthy premenopausal women (age 35–40 years). Bone mineral density (BMD) was measured at the spine and hip.
Results
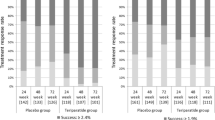
There was a decrease in BTM in response to raloxifene treatment, percentage change at 12 weeks: C terminal telopeptide of type I collagen (CTX) −39 % (95 % CI −48 to −28) and N terminal propeptide of type I procollagen (PINP) −32 % (95 % CI −40 to −23) P < 0.001. The proportion of women classified as responding to treatment using LSC at 12 weeks was as follows: CTX 38 % and PINP 52 % and at 48 weeks CTX 60 % and PINP 65 %. For the RI approach, the proportion of women classified as responding to treatment at 12 weeks was CTX and PINP 38 % and at 48 weeks CTX 40 % and PINP 45 %. There was a significant difference in the change in spine BMD in the raloxifene-treated group compared to the no-treatment group at week 48: difference 0.031 g/cm2 (95 % CI 0.016 to 0.046, P < 0.001).
Conclusions
The two approaches identified women that reached the target for treatment using BTM. Both LSC and RI criteria appear useful in identifying treatment response, but the two approaches do not fully overlap and may be complementary.



Similar content being viewed by others
References
Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, Draper M, Christiansen C (1997) Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med 337:1641–1647
Lufkin EG, Whitaker MD, Nickelsen T, Argueta R, Caplan RH, Knickerbocker RK, Riggs BL (1998) Treatment of established postmenopausal osteoporosis with raloxifene: a randomized trial. J Bone Miner Res 13:1747–1754
Maximov PY, Lee TM, Jordan VC (2013) The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol 8:135–155
Ettinger B, Black DM, Mitlak BH et al (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282:637–645
Bjarnason NH, Sarkar S, Duong T, Mitlak B, Delmas PD, Christiansen C (2001) Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int 12:922–930
Cosman F, Lindsay R (1999) Selective estrogen receptor modulators: clinical spectrum. Endocr Rev 20:418–434
Seeman E, Crans GG, Diez-Perez A, Pinette KV, Delmas PD (2006) Anti-vertebral fracture efficacy of raloxifene: a meta-analysis. Osteoporos Int 17:313–316
Garnero P, Shih WJ, Gineyts E, Karpf DB, Delmas PD (1994) Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab 79:1693–1700
Eastell R, Rogers A, Ni X, Krege JH (2011) Effects of raloxifene and alendronate on bone turnover as assessed by procollagen type I N-terminal propeptide. Osteoporos Int 22:1927–1934
Vasikaran S, Eastell R, Bruyere O et al (2011) Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 22:391–420
Sarkar S, Reginster JY, Crans GG, Diez-Perez A, Pinette KV, Delmas PD (2004) Relationship between changes in biochemical markers of bone turnover and BMD to predict vertebral fracture risk. J Bone Miner Res 19:394–401
Bergmann P, Body JJ, Boonen S, Boutsen Y, Devogelaer JP, Goemaere S, Kaufman JM, Reginster JY, Gangji V (2009) Evidence-based guidelines for the use of biochemical markers of bone turnover in the selection and monitoring of bisphosphonate treatment in osteoporosis: a consensus document of the Belgian Bone Club. Int J Clin Pract 63:19–26
Reginster JY, Sarkar S, Zegels B, Henrotin Y, Bruyere O, Agnusdei D, Collette J (2004) Reduction in PINP, a marker of bone metabolism, with raloxifene treatment and its relationship with vertebral fracture risk. Bone 34:344–351
Clowes JA, Peel NF, Eastell R (2004) The impact of monitoring on adherence and persistence with antiresorptive treatment for postmenopausal osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab 89:1117–1123
Baxter I, Rogers A, Eastell R, Peel N (2013) Evaluation of urinary N-telopeptide of type I collagen measurements in the management of osteoporosis in clinical practice. Osteoporos Int 24:941–947
Bell KJ, Hayen A, Irwig L, Hochberg MC, Ensrud KE, Cummings SR, Bauer DC (2012) The potential value of monitoring bone turnover markers among women on alendronate. J Bone Miner Res 27:195–201
Hannon R, Eastell R (2000) Preanalytical variability of biochemical markers of bone turnover. Osteoporos Int 11(Suppl 6):S30–S44
Seibel MJ, Lang M, Geilenkeuser WJ (2001) Interlaboratory variation of biochemical markers of bone turnover. Clin Chem 47:1443–1450
Schafer AL, Vittinghoff E, Ramachandran R, Mahmoudi N, Bauer DC (2010) Laboratory reproducibility of biochemical markers of bone turnover in clinical practice. Osteoporos Int 21:439–445
Naylor KE, Jacques RM, Paggiosi M, Gossiel F, Peel NF, McCloskey EV, Walsh JS, Eastell R (2016) Response of bone turnover markers to three oral bisphosphonate therapies in postmenopausal osteoporosis: the TRIO study. Osteoporos Int 27:21–31
Paggiosi MA, Peel N, McCloskey E, Walsh JS, Eastell R (2014) Comparison of the effects of three oral bisphosphonate therapies on the peripheral skeleton in postmenopausal osteoporosis: the TRIO study. Osteoporos Int 25:2729–2741
Finigan J, Naylor K, Paggiosi MA, Peel NF, Eastell R (2013) Adherence to raloxifene therapy: assessment methods and relationship with efficacy. Osteoporos Int 24:2879–2886
Levine RJ (1994) Monitoring for adherence: ethical considerations. Am J Respir Crit Care Med 149:287–288
Baim S, Wilson CR, Lewiecki EM, Luckey MM, Downs RW Jr, Lentle BC (2005) Precision assessment and radiation safety for dual-energy X-ray absorptiometry: position paper of the International Society for Clinical Densitometry. J Clin Densitom 8:371–378
Prestwood KM, Gunness M, Muchmore DB, Lu Y, Wong M, Raisz LG (2000) A comparison of the effects of raloxifene and estrogen on bone in postmenopausal women. J Clin Endocrinol Metab 85:2197–2202
Naylor KE, Clowes JA, Finigan J, Paggiosi MA, Peel NF, Eastell R (2010) The effect of cessation of raloxifene treatment on bone turnover in postmenopausal women. Bone 46:592–597
Rosen CJ, Hochberg MC, Bonnick SL et al (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20:141–151
Black DM, Delmas PD, Eastell R et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Vasikaran SD (2008) Utility of biochemical markers of bone turnover and bone mineral density in management of osteoporosis. Crit Rev Clin Lab Sci 45:221–258
Cummings SR, Palermo L, Browner W, Marcus R, Wallace R, Pearson J, Blackwell T, Eckert S, Black D (2000) Monitoring osteoporosis therapy with bone densitometry: misleading changes and regression to the mean. Fracture Intervention Trial Research Group. JAMA 283:1318–1321
Chapurlat RD, Blackwell T, Bauer DC, Cummings SR (2001) Changes in biochemical markers of bone turnover in women treated with raloxifene: influence of regression to the mean. Osteoporos Int 12:1006–1014
Barnett AG, van der Pols JC, Dobson AJ (2005) Regression to the mean: what it is and how to deal with it. Int J Epidemiol 34:215–220
Acknowledgments
This work was supported by Eli Lilly Pharmaceuticals, UK (clinical study), and Immunodiagnostic Systems, UK (providing reagents for iSYS assays).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr Naylor, Miss Gossiel and Dr Jacques have no disclosures. Dr N Peel has received speaker’s honoraria and funding to attend educational events from Warner Chilcott, Lilly, Servier, Merck, Roche, GSK and ProStrakan and consultancy fees from Internis Pharma, ProStrakan and Lilly. Professor Eastell has received funding from the National Institute for Health research (NIHR) and grant/consultancy funding from Warner Chilcott, Eli Lilly, Roche, Immunodiagnostic Systems and Merck.
Rights and permissions
About this article
Cite this article
Naylor, K.E., Jacques, R.M., Peel, N.F.A. et al. Response of bone turnover markers to raloxifene treatment in postmenopausal women with osteopenia. Osteoporos Int 27, 2585–2592 (2016). https://doi.org/10.1007/s00198-016-3573-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3573-z