Abstract
Summary
In the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) study, women with incident clinical fractures reported significant declines in health-related quality of life (HRQoL). The largest declines were observed when the assessment was <3 months post fracture. The largest impact of incident clinical fractures was on physical function, and that of incident clinical vertebral fractures was on back pain.
Introduction
In the FREEDOM trial, denosumab significantly reduced the risk of new vertebral, hip, and nonvertebral fractures. We evaluated the effect of denosumab on HRQoL and the association between incident clinical fractures and HRQoL.
Methods
The FREEDOM trial enrolled 7,868 women aged 60–90 years with a total hip and/or lumbar spine BMD T-score <−2.5 and not <−4.0 at either site. Women were randomized to receive denosumab 60 mg or placebo every 6 months, in addition to daily calcium and vitamin D. HRQoL was assessed with the Osteoporosis Assessment Questionnaire-Short Version (OPAQ-SV) at baseline and every 6 months for 36 months. The OPAQ-SV assesses physical function, emotional status, and back pain. Higher scores indicate better health status.
Results
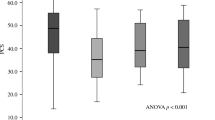
No statistically significant differences in mean change in HRQoL from baseline to end of study were found when comparing treatment groups. Compared with women without any incident fractures during the study, women with incident clinical fractures reported significant declines in physical function (−4.0 vs. −0.5) and emotional status (−5.0 vs. −0.8) at month 36 (P < 0.001 for both). Importantly, time-dependent covariate analyses demonstrated that the largest declines were observed when the assessment was <3 months post fracture. The largest impact of incident clinical fractures was on physical function, and that of incident clinical vertebral fractures was on back pain.
Conclusions
These findings not only demonstrate that incident clinical fractures impact HRQoL but also contribute new information regarding the impact of these fracture events on HRQoL over time.

Similar content being viewed by others
References
Oglesby AK, Minshall ME, Shen W, Xie S, Silverman SL (2003) The impact of incident vertebral and non-vertebral fragility fractures on health-related quality of life in established postmenopausal osteoporosis: results from the teriparatide randomized, placebo-controlled trial in postmenopausal women. J Rheumatol 30:1579–1583
Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, Kanis J (2000) Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res 15:1384–1392
Salaffi F, Cimmino MA, Malavolta N, Carotti M, Di Matteo L, Scendoni P, Grassi W (2007) The burden of prevalent fractures on health-related quality of life in postmenopausal women with osteoporosis: the IMOF study. J Rheumatol 34:1551–1560
Silverman SL, Minshall ME, Shen W, Harper KD, Xie S (2001) The relationship of health-related quality of life to prevalent and incident vertebral fractures in postmenopausal women with osteoporosis: results from the Multiple Outcomes of Raloxifene Evaluation Study. Arthritis Rheum 44:2611–2619
Brenneman SK, Barrett-Connor E, Sajjan S, Markson LE, Siris ES (2006) Impact of recent fracture on health-related quality of life in postmenopausal women. J Bone Miner Res 21:809–816
Marquis P, Roux C, de la Loge C, Diaz-Curiel M, Cormier C, Isaia G, Badurski J, Wark J, Meunier PJ (2008) Strontium ranelate prevents quality of life impairment in post-menopausal women with established vertebral osteoporosis. Osteoporos Int 19:503–510
Nevitt MC, Thompson DE, Black DM, Rubin SR, Ensrud K, Yates AJ, Cummings SR (2000) Effect of alendronate on limited-activity days and bed-disability days caused by back pain in postmenopausal women with existing vertebral fractures. Fracture Intervention Trial Research Group. Arch Intern Med 160:77–85
Madureira MM, Bonfa E, Takayama L, Pereira RM (2010) A 12-month randomized controlled trial of balance training in elderly women with osteoporosis: improvement of quality of life. Maturitas 66:206–211
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Shen W, Silverman SL, Minshall ME, Harper KD, Xie S (1999) Measuring health-related quality of life in postmenopausal women with osteoporosis: development and validation of a short version of the osteoporosis assessment questionnaire. J Bone Miner Res 14:S204–S204
Silverman SL, Cranney A (1997) Quality of life measurement in osteoporosis. J Rheumatol 24:1218–1221
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Randell AG, Bhalerao N, Nguyen TV, Sambrook PN, Eisman JA, Silverman SL (1998) Quality of life in osteoporosis: reliability, consistency, and validity of the Osteoporosis Assessment Questionnaire. J Rheumatol 25:1171–1179
Norman GR, Sloan JA, Wyrwich KW (2003) Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 41:582–592
Revicki D, Hays RD, Cella D, Sloan J (2008) Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol 61:102–109
Wolfe F, Michaud K, Li T, Katz RS (2010) Chronic conditions and health problems in rheumatic diseases: comparisons with rheumatoid arthritis, noninflammatory rheumatic disorders, systemic lupus erythematosus, and fibromyalgia. J Rheumatol 37:305–315
Marsh JL, McKinley T, Dirschl D, Pick A, Haft G, Anderson DD, Brown T (2010) The sequential recovery of health status after tibial plafond fractures. J Orthop Trauma 24:499–504
Hagino H, Nakamura T, Fujiwara S, Oeki M, Okano T, Teshima R (2009) Sequential change in quality of life for patients with incident clinical fractures: a prospective study. Osteoporos Int 20:695–702
Acknowledgments
Amgen Inc. sponsored this study. The authors would like to thank Michelle Geller, MD; Andreas Grauer, MD; Luanda Grazette, MD; and Cesar Libanati, MD, for their contribution toward the development of the comorbidity index, and Michelle N. Bradley, PhD, who provided medical writing support on behalf of Amgen Inc.
Conflicts of interest
This study was funded by Amgen Inc. S. Silverman is a consultant, speaker, and/or research investigator for Amgen Inc., Eli Lilly, Merck, Novartis, Procter & Gamble, Roche Diagnostics, Roche Pharmaceuticals, and Wyeth. H. N. Viswanathan, Y-C Yang, A. Wang, and D. Macarios are employees and shareholders of Amgen Inc. S. Boonen is a consultant for Amgen Inc. S. Ragi-Eis is a consultant, advisory board member, speaker, and/or research investigator for Amgen Inc., Merck, Novartis, Pfizer, Roche, and Sanofi–Aventis. P. Fardellone, N. Gilchrist, S. Palacios, and K. Pavelka are investigators for Amgen Inc. P. Lips is a speaker and/or research investigator for Amgen Inc., Eli Lilly, Merck, Servier, and Wyeth. M. Nevitt and D. Revicki are consultants and investigators for Amgen Inc. J. Simon is a consultant, advisory board member, speaker, and/or research investigator for Allergan, The Alliance for Better Bone Health, Amgen Inc., Ascend Therapeutics, Barr, Bayer, BioSante, Boehringer Ingelheim, Corcept Therapeutics, FemmePharma, GSK, KV Pharmaceutical, Meditrina Pharmaceuticals, Merck, Merrion Pharmaceuticals, Nanma/Tripharma/Trinity, Novartis, Novo Nordisk, Novogyne, Pear Tree Pharmaceuticals, Procter & Gamble, QuatRx Pharmaceuticals, Roche, Schering-Plough, Sciele, Solvay, Warner Chilcott, and Wyeth. E. Siris is a consultant, advisory board member, and/or speaker for Amgen, Eli Lilly, Merck, Novartis, and Pfizer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Clinicaltrials.gov
NCT00089791; August 13, 2004
Financial support
This study was sponsored by Amgen Inc., Thousand Oaks, CA, USA.
Rights and permissions
About this article
Cite this article
Silverman, S., Viswanathan, H.N., Yang, YC. et al. Impact of clinical fractures on health-related quality of life is dependent on time of assessment since fracture: results from the FREEDOM trial. Osteoporos Int 23, 1361–1369 (2012). https://doi.org/10.1007/s00198-011-1720-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1720-0