Abstract
Summary
In this meta-analysis of all Merck-conducted, placebo-controlled clinical trials of alendronate, the occurrence of AF was uncommon, with most studies reporting two or fewer events. Across all studies, no clear association between overall bisphosphonate exposure and the rate of serious or non-serious AF was observed.
Introduction
To explore the incidence of atrial fibrillation (AF) and other cardiovascular endpoints in clinical trials of alendronate.
Methods
All double-blind, placebo-controlled studies of alendronate 5, 10, or 20 mg daily, 35 mg once-weekly, 35 mg twice-weekly, and 70 mg once-weekly of at least 3 months duration conducted by Merck were included in this meta-analysis. The primary method of analysis was exact Poisson regression. Estimated relative risk (RR) of alendronate versus placebo and the associated 95% confidence interval was derived from a model that included number of episodes with factors for treatment group and study and an offset parameter for number of person-years on study.
Results
Of 41 studies considered, 32 met all criteria for inclusion in the analysis (participants—9,518 alendronate, 7,773 placebo). Estimated RR for all AF events was 1.16 (95% CI = 0.87, 1.55; p = 0.33). Most trials had two or fewer AF events. The RR of AF classified as a serious adverse event was 1.25 (95% CI = 0.82, 1.93; p = 0.33), but became 0.97 (95% CI = 0.51, 1.85) when the clinical fracture cohort of the Fracture Intervention Trial was excluded, indicating that results were driven by events in that study. Estimated RRs for other cardiovascular endpoints were less than 1.
Conclusions
The incidence of atrial fibrillation was low in Merck clinical trials of alendronate and was not significantly increased in any single trial nor in the meta-analysis. Based on this analysis, alendronate use does not appear to be associated with an increased risk of atrial fibrillation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation is the most common sustained cardiac arrhythmia, affecting more than 2 million individuals in the USA [1, 2]. Because the population is aging and age 65 or greater is a strong risk factor for AF, the prevalence of AF is expected to increase to nearly 16 million cases by 2050 [2]. Extrapolation from Framingham cohort data suggests one in four adults will experience at least one episode of AF in their lifetime [3].
Bisphosphonates are the most widely used class of drugs for the treatment of osteoporosis. Black et al. [4] reported an increased risk of serious atrial fibrillation (AF) adverse experiences (SAEs) in a study of once-yearly intravenous zoledronic acid for the treatment of postmenopausal osteoporosis. In that study, the number of participants with AF SAEs was significantly greater with zoledronic acid than with placebo [50 (1.3%) vs. 20 (0.5%) participants, p < 0.001]. As noted in a letter to the editor by Cummings et al., published concurrently, there was a nominally but not significantly increased risk of AF SAEs with alendronate, an oral bisphosphonate, for participants in the Fracture Intervention Trial (FIT) [Relative Risk (RR) = 1.51, 95% CI = 0.97, 2.40, p = 0.07 for AF SAEs for alendronate compared with placebo; RR = 1.14, 95% CI = 0.83, 1.57, p = 0.42 for all (serious and non-serious) AF AEs] [5]. Since these two reports, others have conducted meta-analyses of data from ibandronate clinical trials as well as from the published literature and from retrospective studies to examine the risk for AF in patients taking bisphosphonates for the treatment of osteoporosis [6–11]. The report of an increased risk of AF with zoledronic acid and the observations regarding the original alendronate FIT data prompted us to explore, using both published and unpublished data, the incidence of AF and other related cardiovascular (CV) endpoints with alendronate compared with placebo in clinical trials conducted by Merck. In addition to the meta-analysis, information is summarized on myocardial infarctions (MIs) and CV deaths from the FIT trial, the only trial to adjudicate CV AEs.
Methods
Objective
The primary objective of this meta-analysis was to explore the incidence of AF (atrial fibrillation or atrial flutter) AEs for participants in alendronate clinical trials and to compare the relative risk of these events between alendronate-treated and placebo-treated participants. Secondary objectives were to explore the incidence of all cardiac arrhythmias, non-hemorrhagic cerebrovascular accidents (CVA), and congestive heart failure (CHF) in these clinical trials and to compare the relative risk of these events between alendronate-treated and placebo-treated participants. In addition, the possible association of alendronate with MI and CV death in FIT, the only trial with adjudicated CV events, was explored.
Analyses
All the analyses in this study were predefined. There was a full meta-analysis protocol prepared and approved by all authors before any analyses were conducted. Each participant experiencing an endpoint was only counted once for that endpoint; however, participants with more than one type of endpoint could be counted separately for each endpoint. All events of AF reported as AEs by the study investigator were included in the analysis. All events of AF and other cardiac arrhythmias reported for FIT were adjudicated at the time of the study by a physician blinded to treatment allocation; a data and safety monitoring committee reviewed the unblinded safety data periodically throughout the trial. Cardiac arrhythmia and AF event data from all other studies were reported as AEs without additional adjudication. AEs were classified as serious if they met the regulatory definition of a “serious” AE as reported by the study investigator. For these studies, an SAE was defined as any AE that results in death, is life threatening, results in a persistent or significant disability/incapacity, results in or prolongs an existing hospitalization, is a congenital anomaly/birth defect (in offspring of patient), is a cancer, or is an overdose (whether accidental or intentional). Events included both new events in participants with no prior history of AF and worsening events (i.e., recurrent AF or increasing clinical signs/symptoms in participants with chronic AF). To insure complete accounting, AEs of atrial fibrillation and the closely related AEs of atrial flutter are grouped as the primary endpoint of the analysis.
Other endpoints that were explored due to their potential association with AF were the incidence of all cardiac arrhythmias, non-hemorrhagic CVA, and CHF (see Online supplement for terms used to identify events).
Choice of studies and treatment groups
All Merck-conducted, double-blind, placebo-controlled studies of alendronate 5 mg daily, 10 mg daily, 20 mg daily, 35 mg once-weekly, 35 mg twice-weekly, and 70 mg once-weekly of at least 3 months duration were included in this analysis (Table 1); the few short duration trials were clinical pharmacology studies without a placebo comparator, and none had any AF events. Treatment groups with daily doses of <5 mg were excluded because the lower-dose studies could bias toward the null even if there were a true causal relationship. Treatment groups with daily doses >20 mg were also excluded. Only studies conducted by Merck or for Merck by a contract research organization were included. Extension studies were included for the AE analysis if participants were still blinded to treatment allocation and remained on the same treatment and if there was a placebo group for comparison. In FLEX, the long-term extension of FIT, participants from FIT, after an average of 5 years of prior alendronate therapy, were randomized to one of three treatment arms for an additional 5 years: 10 mg alendronate, 5 mg alendronate, or placebo. Although FLEX was not included in the meta-analysis, because all participants had previously received alendronate for ~5 years, data for AF AEs in FLEX are summarized separately because of the large patient population. For each study included in the analysis, all study groups with doses of alendronate within the pre-specified range were combined to form a single pooled “alendronate” group. Changes of alendronate dose within the pre-specified range were not distinguished. All participants treated with placebo following active treatment or active treatment following placebo were included until the change of treatment. The two cohorts of FIT, the vertebral fracture cohort (identified as study 51.1) and the clinical fracture cohort (identified as study 51.2), were two trials within a single protocol, but were analyzed as two separate studies.
Statistical methods
The studies included in this meta-analysis span several years, and data from different studies were collected using different methods and databases. Because of this, patient-level time-to-event data were not always available to conduct the analyses described here. Meta-analysis was used to calculate a weighted average from the individual studies. The primary method of analysis for all endpoints was exact Poisson regression. An estimate for the relative risk of alendronate versus placebo and the associated 95% confidence interval (CI) was derived from a model that included the number of episodes with factors for treatment group and study and an offset parameter for the number of person-years on study. The exact number of person-years of follow-up for each treatment group within each trial was calculated using patient-level information utilizing the first and last treatment date on study drug. The relative risk and associated confidence intervals were reported for each study from the exact Poisson regression model with a factor for treatment. When zero events occurred in the placebo group, the relative risk for the study was undefined and could not be calculated. In isolated cases, the statistical analysis procedure could not calculate confidence intervals for the relative risk due to the absence of events; in those cases, the relative risk alone was reported as a summary statistic.
The odds ratio was reported from a fixed-effects meta-analysis model using Mantel–Haenszel methods with a Robins–Breslow–Greenland variance. A continuity correction factor (CCC), to account for studies with zero events, was added to the placebo cells, and a treatment correction factor (TCC) was added to the alendronate cells in each cell of the 2 × 2 table, proportional to the reciprocal of the other treatment group and such that TCC + CCC = 0.01 [12]. The odds ratio was reported for each study and could not be calculated when zero events occurred in the placebo group. When zero events occurred only in the alendronate group of the study, the odds ratio was zero. Both the relative risk and the odds ratio were reported to provide a more complete perspective of the data set.
A test for heterogeneity was conducted using the treatment-by-study interaction term in exact Poisson regression model. The stability of the estimates was evaluated by conducting exact Poisson regression meta-analysis with each study eliminated one at a time and by constructing estimates within pre-specified subgroups as below:
-
1.
Age: Average study age ≤65, >65 years
-
2.
Elderly participants (mean age of 70 years) (yes, no): Elderly study—Protocol 054 (mean age 70.8 years), FIT vertebral fracture study—Protocol 51.1 (mean age 70.8 years), Nursing home study—Protocol 087 (mean age 78.5 years) vs. all other studies (mean age 68.5 years)
-
3.
Studies for the prevention of osteoporosis (Protocols 029, 038, and 055) were grouped together. A second group comprised protocols 035, 037 (the original Phase III studies), and 051 (Phase III study for the subsequent fracture endpoint), all similarly designed long-term studies for the treatment of osteoporosis rather than prevention. All other studies comprised the third group.
-
4.
Length of study: ≤1 year, >1 year
These meta-analyses are exploratory in nature. No multiplicity adjustments were made.
Assuming an incidence rate of five per 1,000 person-years (the incidence observed in the placebo group), the 18,000 person-years in the two treatment groups is sufficient to detect a 50% increase in the alendronate group with more than 90% power assuming a one-sided significance level or 85% power assuming a two-sided significance level. The 18,000 person-years in the two treatment groups is sufficient to detect a 40% increase in the alendronate group with more than 75% power assuming a one-sided significance level.
Supplemental analyses in FIT
Additional post hoc analyses were performed in FIT to further evaluate MI SAEs. Post hoc subgroup analyses of this nature should be interpreted with caution because the possibility of chance findings increases whenever multiple analyses are performed. In this analysis, the investigators’ original reported diagnosis was included by default in cases where the adjudicated consensus was “insufficient data.” Primary intention-to-treat analyses were applied to adjudicated data. It was pre-specified that p values would not be provided for adjudicated data, based on statistical issues concerning potential misinterpretation in the context of a post hoc assessment of this nature. Consequently, only relative risks and 95% CIs are reported.
Results
Forty-one studies were considered for the meta-analysis. Thirty-two studies met all criteria for inclusion in the analysis, including having alendronate participant groups within the pre-specified dose range for alendronate (Table 1). The 32 studies represent 9,518 participants and 20,265 person-years on alendronate, with an average of 2.13 person-years per subject, and 7,773 participants and 18,018 person-years on placebo, with an average of 2.32 person-years per subject. Follow-up time ranged from 12 weeks for Studies 162 and 904 to 6 years for study 055.
Endpoint of atrial fibrillation or atrial flutter
All AF events (atrial fibrillation and atrial flutter)
The p value for the test for heterogeneity was 0.30 based on the treatment-by-study interaction term in the Poisson regression model. The estimated relative risk for all events of AF (serious and non-serious combined) was 1.16 (95% CI = 0.87, 1.55; p = 0.33; Fig. 1A) and was similar to the estimated odds ratio for all events: 1.16 (95% CI = 0.87, 1.53; p = 0.32; Table 2). There were 112 events of AF reported in the 9,518 participants taking alendronate included in the analysis, occurring in 17 trials. Eighty-nine events were reported in the 7,773 participants taking placebo, occurring in 12 trials. In 24 trials, there were fewer than two AF events in either treatment group; of these, 11 trials (34.4%) did not have any reported events of AF. Results for atrial fibrillation without including atrial flutter were similar, with only five events on alendronate and three events on placebo attributed to atrial flutter alone (data not shown). At the end of FLEX, there were eight AF events with 1,398.6 patient-years in the 10-mg arm, 10 AF events with 1,397.7 patient-years in the 5-mg arm, and 10 AF events with 1,837.7 patient-years in the placebo arm.
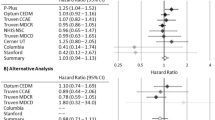
Relative risk (RR) of all events (A) or serious events (B) of atrial fibrillation or flutter in placebo-controlled trials of alendronate conducted by Merck. Study 51.1 is the vertebral fracture cohort of FIT, and study 51.2 is the clinical fracture cohort of FIT. Undefined indicates that there were no AF events in the placebo arm of the study, although there may have been an event in the alendronate arm. 0.00 indicates that there were no AF events in the alendronate arm and at least one AF event in the placebo arm
Serious AF events
The p value for the test for heterogeneity was 0.13 based on the treatment-by-study interaction term in the Poisson regression model. The estimated relative risk for AF SAEs was 1.25 (95% CI = 0.82, 1.93; p = 0.33, Fig. 1B) and was similar to the estimated odds ratio for all serious events of 1.24 (95% CI = 0.87, 1.87; p = 0.29; Table 2). There were 55 participants with one or more AF SAEs for alendronate occurring in six trials compared with 41 events for placebo occurring in eight trials. Twenty-two trials (68.8%) did not have any AF SAEs. Results for atrial fibrillation without including atrial flutter were similar (data not shown).
Sensitivity analysis
The stability of the estimates for all events and for SAEs was evaluated by conducting exact Poisson regression meta-analyses with each study eliminated one at a time. The order of magnitude of the relative risk for all events of AF changed very little as each study was eliminated, although the 95% confidence interval became wider when the large clinical fracture cohort of FIT, study 51.2, was eliminated (Fig. 2A).
Relative risk (RR) of all events (A) or serious events (B) of atrial fibrillation or atrial flutter cross-validation by eliminating one study at a time. For example, the first RR represents all trials except study 26, etc. Study 51.1 is the vertebral fracture cohort of FIT, and study 51.2 is the clinical fracture cohort of FIT
The two cohorts for FIT, which represent 34% of the participants taking alendronate and 41% of the participants taking placebo, experienced 87.3% of the AF SAEs for alendronate and 78.0% of the AF SAEs for placebo. The relative risk of AF SAEs including all studies was 1.25 (95% CI = 0.82, 1.93), but became 0.97 (95% CI = 0.51, 1.85) when the clinical fracture cohort of FIT, study 51.2, was excluded (Fig. 2B), indicating that the results for serious events were driven by the AF SAEs in that FIT cohort [RR 1.56 (95% CI = 0.86, 2.89) for AF SAEs in the clinical fracture cohort]. In the vertebral fracture cohort (study 51.1), the relative risk of AF SAEs was 1.37 (95% CI = 0.62, 3.15), but this cohort had a smaller contribution to the overall results because it represented approximately one third of the patient years of the clinical fracture cohort.
Figure 3 summarizes the relative risk of AF and serious events of AF within the pre-specified subgroups. Both cohorts for FIT are included in the >65 group for age, length of study >1 year, and pivotal studies of osteoporosis. The clinical fracture cohort of FIT is not included in the elderly participants group because the average age was less than 70 years old (mean age 61 years). The results of the clinical fracture cohort of FIT overwhelm the results of the other studies to the extent that the subgroup analyses reflect the presence or absence of that cohort in the subgroup.
Other endpoints
The endpoints of CA, CVA, and CHF were examined in the meta-analysis using the same studies and the same patient populations as were used for the atrial fibrillation endpoint: 32 trials including 9,518 participants on alendronate and 7,773 on placebo.
Cardiac arrhythmias
The estimated relative risk for all AEs of cardiac arrhythmia (including AF) was 0.92 (95% CI = 0.79, 1.07; p = 0.31), and the estimated odds ratio was 0.91 (95% CI = 0.78, 1.06; p = 0.23). The estimated relative risk for SAEs was 1.18 (95% CI = 0.87, 1.61; p = 0.31), and the estimated odds ratio was 1.17 (95% CI = 0.87, 1.59; p = 0.30). There were 360 AEs and 98 SAEs of cardiac arrhythmia for alendronate, occurring in 26 trials (Online Table A). There were 346 AEs and 78 SAEs of cardiac arrhythmia for placebo, occurring in 24 trials. Thirty trials had at least one event in either treatment group; two trials had no events. As seen with the AF endpoint, FIT accounted for two thirds of the arrhythmia events (study 51.1—alendronate = 85, placebo = 78, RR = 1.06; study 51.2—alendronate = 159, placebo = 162, RR = 0.99).
Non-hemorrhagic cerebrovascular accidents (CVA)
The estimated relative risk for all CVA AEs was 0.85 (95% CI = 0.65, 1.11; p = 0.25), and the estimated odds ratio was 0.84 (95% CI = 0.65, 1.10; p = 0.21). There were 108 CVA AEs for alendronate occurring in 11 trials, compared with 122 CVA AEs for placebo occurring in nine trials (Online Table A). Thirteen trials had CVA AEs; 19 trials had no CVA events.
Congestive heart failure (CHF)
The estimated relative risk for all CHF AEs was 0.96 (95% CI = 0.71, 1.30; p = 0.84), and the estimated odds ratio was 0.95 (95% CI = 0.71, 1.28; p = 0.75). There were 91 CHF AEs for alendronate occurring in 11 trials compared with 91 AEs for placebo occurring in eight trials (Online Table A). Thirteen trials had an AE in one or both treatment groups; 19 trials had no CHF events.
Myocardial infarctions and cardiovascular deaths in FIT
As FIT was the largest trial included in this meta-analysis and as it was the only trial to adjudicate CV AEs, only MIs and CV deaths from FIT are summarized. An analysis of the adjudicated results of all FIT SAEs attributed to coronary heart disease (CHD) in the combined cohort did not demonstrate a significant increase in risk of MI with alendronate compared with placebo (1.4% vs. 1.1%, RR 1.28, 95% CI = 0.82, 2.00). All CV deaths that occurred during FIT, as well as all deaths reported with the term “sudden death,” were included in the adjudication. There were 23 CV deaths in the placebo group and 28 in the alendronate group [RR = 1.22 (95% CI = 0.68, 2.21), p = 0.578 for alendronate vs. placebo]. Subgroups in CV deaths were sudden/unknown (placebo = 8, alendronate = 9), fatal MI (placebo = 3, alendronate = 6), cardiac non-myocardial infarction, defined as an event unrelated to myocardial ischemia (placebo = 1, alendronate = 7), and non-cardiac (cardiovascular) (placebo = 11, alendronate = 6). The number of deaths in the different subcategories was too small to allow meaningful conclusions.
Discussion
In this meta-analysis of all Merck-conducted, placebo-controlled clinical trials of alendronate, the occurrence of AF was uncommon, with most studies reporting two or fewer events. Across all studies, no clear association between overall bisphosphonate exposure and the rate of serious or non-serious AF was observed.
The present study included published and unpublished data from all trials of alendronate of at least 3 months duration meeting eligibility criteria selected prior to analyses. The total number of individuals in the smaller, shorter studies was similar to the total number enrolled in FIT, permitting the comparison most relevant to determining whether AF was caused by the study medication or was a chance association.
The analysis of rare event data is problematic. Poisson regression, the method used here, assumes a constant hazard rate over time, within each study. Given the small number of events, the appropriateness of this assumption within these studies would be hard to evaluate. Based on a review of AF in FIT and the incidence of AF SAEs in the HORIZON zoledronic acid trial, which were reported to have occurred uniformly over time, the assumption of a constant hazard rate over time is reasonable, however, and the summary measure of the event rate per patient-year of follow-up for each trial appears to be appropriate. In addition, most commonly used methods of meta-analysis (log-odd or log risk ratio) become undefined when zero events occur in either or both groups of a study [13, 14]. Standard statistical software either eliminates these studies completely or introduces correction factors that seriously bias the results, but there is information to be gained about absolute risks by including large or long-running studies without any events.
The results of the current meta-analysis are in accord with the findings of the FDA regarding all bisphosphonates, which concluded that the incidence of AF was rare in clinical trial data and that there was no clear association between overall bisphosphonate exposure and the rate of serious or non-serious atrial fibrillation [15]. Others who have looked at the incidence of AF in bisphosphonate trials since the initial reports by Black et al. [4] and Cummings and colleagues [5] have reported no association, including in a second trial of intravenous zolendronate [6–11]. Lewiecki et al. [10] analyzed pooled data from the four pivotal trials of ibandronate and found no increased risk of AF with any ibandronate regimen. Loke et al. [11] conducted a systematic review of four datasets from placebo-controlled RCTs and two case–control studies of bisphosphonates and found no association of overall AF with bisphosphonate use, but a modest association of AF SAEs with use, driven by one of the zolendronic acid (HORIZON) trials and the alendronate (FIT) trial. Although some retrospective epidemiologic studies have seen evidence of an increased risk of AF with bisphosphonate use [16–18], others have found that long-term risk of AF with bisphosphonates did not differ from risk with raloxifene use [19] or with no bisphosphonate use [20–22]. Vestergaard et al. examined the effect of heart disease and lung disease on the association between oral bisphosphonate use and AF in a cohort study using the Danish National Hospital Discharge Register and found that any excess risk of AF became non-significant when chronic obstructive pulmonary disease was introduced as a confounder [23].
In the present analysis, the FIT clinical fracture cohort is the only trial of oral alendronate that suggested a potential increased risk of serious AF [p = 0.07; 47 events (1.5%) for alendronate and 31 events (1.0%) for placebo over an average of 4 years]. FIT was among the largest, longest oral bisphosphonate trials and the only trial that prospectively adjudicated all cases of AF. FIT had approximately the same number of subjects as all other trials combined. Further analyses of the data from the combined cohort of FIT showed that all (serious plus non-serious) AF AEs, as well as all arrhythmia AEs, were approximately balanced between the groups, making the possibility of a true association between AF and alendronate treatment unlikely.
It is not surprising that osteoporosis and AF occur together in the elderly, as the prevalence of both increases with age. Individuals with osteoporosis tend to be older and have more cardiovascular disease, which may contribute to the appearance of an increased risk of AF with bisphosphonate treatment seen in observational studies [16, 19, 22, 24, 25].
Overall, our data do not support a causal relationship between alendronate and AF, as a (non-significant) trend was observed in only a single randomized alendronate clinical study. Furthermore, there is no plausible mechanism for such an association. There was no clear evidence that oral bisphosphonates caused calcium/electrolyte imbalance in the blood (e.g., hypocalcemia), a hypothetical mechanism proposed by Heckbert et al. [16], or any other clinical AE that is a known risk factor for AF. There has been speculation about other potential mechanisms [26, 27]. For example, AF and CHF are commonly co-existent conditions that can contribute to the de novo development or worsening of the other [28], but there does not appear to be any evidence for an excess of heart failure in the bisphosphonate-treated population.
Examination of other CV endpoints in the current meta-analysis showed that there were no significant differences in the risk of serious or all (serious plus non-serious) AEs between the placebo and alendronate groups. These results are similar to those found in FIT, which showed that other AEs related to embolic or thrombotic disease, MIs and CV deaths, were generally either evenly distributed or, in some cases, occurred at higher frequency in participants on placebo versus alendronate.
There are some limitations to this meta-analysis. Trial-level data from multiple studies were pooled retrospectively for analysis. Although performing a pooled analysis of individual patient data would have been optimal had it been available, two groups have shown that summary estimates obtained from trial-level aggregated data and pooled individual patient data appear to be equivalent when based on the same studies under the same assumptions [29, 30]. Many CV AEs were adjudicated only in FIT. In the other trials, the recorded AEs were extracted from investigator reports of AEs in each study and are subject to reporting bias. Standard regulatory definitions of “serious” AEs were applied in all cases; however, the application of the “serious” rating may be subjective when there were multiple potentially “serious” AEs associated with a hospitalization and was dependent on the individual blinded investigator’s judgment.
In summary, the incidence of atrial fibrillation was uncommon in these older participants in clinical trials of alendronate and did not differ significantly between alendronate and placebo groups. Based on this analysis, alendronate use did not show evidence of an increased risk of atrial fibrillation.
References
Go AS, Hylek EM, Phillips KA et al (2001) Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 285:2370–2375
Miyasaka Y, Barnes ME, Gersh BJ et al (2006) Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 114:119–125
Lloyd-Jones DM, Wang TJ, Leip EP et al (2004) Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 110:1042–1046
Black DM, Delmas PD, Eastell R et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Cummings SR, Schwarz AV, Black DM (2007) Alendronate and atrial fibrillation. N Engl J Med 356:1895–1896
Karam R, Camm J, McClung M (2007) Yearly zoledronic acid in postmenopausal osteoporosis. N Engl J Med 357:712–713
Lyles KW, Colón-Emeric CS, Magaziner JS et al (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799–1809
Mak A, Cheung MW, Ho RC, Cheak AA, Lau CS (2009) Bisphosphonate and atrial fibrillation: Bayesian meta-analyses of randomized controlled trials and observational studies. BMC Musculoskelet Disord 10:113
Camm AJ (2010) Review of the cardiovascular safety of zoledronic acid and other bisphosphonates for the treatment of osteoporosis. Clin Therap 32:426–436
Lewiecki EM, Cooper C, Thompson E et al (2010) Ibandronate does not increase risk of atrial fibrillation in analysis of pivotal clinical trials. Int J Clin Pract 64:821–826
Loke YK, Jeevanantham V, Singh S (2009) Bisphosphonates and atrial fibrillation: systematic review and meta-analysis. Drug Saf 32:219–228
Sweeting MJ, Sutton AJ, Lambert PC (2004) What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 23:1351–1375
Bradburn MJ, Deeks JJ, Berlin JA, Localio AR (2007) Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 26:53–77
Sutton AJ, Cooper NJ, Lambert PC et al (2002) Meta-analysis of rate and adverse event data. Exp Rev Pharmacoeconomics Outcomes Res 2:367–379
US Food and Drug Administration (2008) Update of safety review follow-up to the October 1, 2007 early communication about the ongoing safety review of bisphosphonates. Available at http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm136201.htm. Accessed 5 November 2009
Heckbert SR, Li G, Cummings SR et al (2008) Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med 168:826–831
Abrahamsen B, Eiken P, Brixen K (2009) Atrial fibrillation in fracture patients treated with oral bisphosphonates. J Intern Med 265:581–592
Bhuriya R, Singh M, Molnar J et al (2010) Bisphosphonate use in women and the risk of atrial fibrillation: a systematic review and meta-analysis. Int J Cardiol 142:213–217
Huang WF, Tsai Y-W, Wen Y-W et al (2010) Osteoporosis treatment and atrial fibrillation: alendronate versus raloxifene. Menopause 17:57–63
Bunch TJ, Anderson JL, May HT et al (2009) Relation of bisphosphonate therapies and risk of developing atrial fibrillation. Am J Cardiol 103:824–828
Grosso A, Douglas I, Hingorani A et al (2009) Oral bisphosphonates and risk of atrial fibrillation and flutter in women: a self-controlled case-series safety analysis. PLoS ONE 4:e4720
Sørensen HT, Christensen S, Mehnert F et al (2008) Use of bisphosphonates among women and risk of atrial fibrillation and flutter: population based case–control study. BMJ 336:813–816
Vestergaard P, Schwartz K, Pinholt EM et al (2010) Risk of atrial fibrillation associated with use of bisphosphonates and other drugs against osteoporosis: a cohort study. Calcif Tissue Int 86:335–342
Recker RR, Lewiecki EM, Miller PD, Reiffel J (2009) Safety of bisphosphonates in the treatment of osteoporosis. Am J Med 122(2 Suppl):S22–S32
von der Recke P, Hansen MA, Hassager C (1999) The association between low bone mass at the menopause and cardiovascular mortality. Am J Med 106:271–278
Pazianas M, Compston J, Huang CL-H (2010) Atrial fibrillation and bisphosphonate therapy. J Bone Miner Res 25:2–10
Kemeny-Suss N, Kasneci A, Rivas D et al (2010) Alendronate affects calcium dynamics in cardiomyocytes in vitro. Vascul Pharmacol 51:350–358
Morrison TB, Bunch TJ, Gersh BJ (2009) Pathophysiology of concomitant atrial fibrillation and heart failure: implications for management. Nat Clin Pract Cardiovasc Med 6:46–56
Olkin I, Sampson A (1998) Comparison of meta-analysis versus analysis of variance of individual patient data. Biometrics 54:317–322
Mathew T, Nordstrom K (1999) On the equivalence of meta-analysis using literature and using individual patient data. Biometrics 55:1221–1223
Acknowledgments
The authors thank Sheng Zhang and Lina Li for programming support, Amy Lamotta and Adela Maragoto for gathering the required information for the alendronate trials, and Jennifer Pawlowski for formatting and submission of the manuscript.
Conflicts of interest
Elizabeth Barrett-Connor, as corresponding author, had full access to all the data included in the meta-analysis and had final responsibility for the decision to submit for publication. All authors met the ICJME criteria for authorship and were involved in at least one of the following: conception, design, acquisition, analysis, statistical analysis, interpretation of data, drafting the manuscript, and/or revising the manuscript for important intellectual content. All authors provided final approval of the version to be published.
Elizabeth Barrett-Connor: I declare that I participated in the conception and design of the meta-analysis, participated in the interpretation of the results and the writing of the initial and subsequent drafts, and that I have seen and approved the final version. I have the following conflicts of interest: received research support from Merck, Arena Pharmaceuticals, Roche, and Pfizer.
Arlene S. Swern: I declare that I participated in the planning and design of the meta-analysis, assembled the data, performed analyses, interpreted the results, provided substantive suggestions for revision on iterations of the draft manuscript, and that I have seen and approved the final version. I have the following conflicts of interest: former employee of Merck who owns stock in the Company.
Henry G. Bone: I declare that I participated in the conception and design of the meta-analysis, participated in the interpretation of the results and the writing of the initial and subsequent drafts, and that I have seen and approved the final version. I have the following conflicts of interest: served as a scientific advisor or consultant to Amgen, Merck, Zelos, Pfizer, GlaxoSmithKline, Novartis, Osteologix, Nordic Bioscience/Sanos, and Takeda Pharmaceuticals and received research support from Amgen, Merck, Zelos, Eli Lilly, Novartis, Nordic Bioscience, and Takeda Pharmaceuticals.
Uri A. Liberman: I declare that I participated in the conception and design of the meta-analysis, participated in the interpretation of the results and the writing of the initial and subsequent drafts, and that I have seen and approved the final version. I have the following conflicts of interest: served on the speakers bureau for Merck.
Socrates Papapoulos: I declare that I participated in the conception and design of the meta-analysis, participated in the interpretation of the results and the writing of the initial and subsequent drafts, and that I have seen and approved the final version. I have the following conflicts of interest: served as a scientific advisor or consultant to Amgen, Merck, Novartis, Procter & Gamble, Roche/GSK, and received research support from Procter & Gamble.
Hongwei Wang: I declare that I participated in the planning and design of the study, assembled the data, performed analyses, interpreted the results, provided substantive suggestions for revision on iterations of the draft manuscript, and that I have seen and approved the final version. I have the following conflicts of interest: former employee of Merck who may own stock in the Company.
Carolyn M. Hustad: I declare that I participated in the interpretation of the results, wrote sections of the initial draft, provided substantive suggestions for revision on iterations of the draft manuscript, and that I have seen and approved the final version. I have the following conflicts of interest: employee of Merck Sharpe & Dohme Corp. who owns stock and holds stock options in the Company.
Anne de Papp: I declare that I participated in the interpretation of the results, provided substantive suggestions for revision on iterations of the draft manuscript, and that I have seen and approved the final version. I have the following conflicts of interest: employee of Merck Sharpe & Dohme Corp. who owns stock and holds stock options in the Company.
Arthur C. Santora: I declare that I participated in the conception, planning, and design of the meta-analysis, interpreted the results, provided substantive suggestions for revision on iterations of the draft manuscript, and that I have seen and approved the final version. I have the following conflicts of interest: employee of Merck Sharpe & Dohme Corp. who owns stock and holds stock options in the Company.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Online supplement (DOC 260 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Barrett-Connor, E., Swern, A.S., Hustad, C.M. et al. Alendronate and atrial fibrillation: a meta-analysis of randomized placebo-controlled clinical trials. Osteoporos Int 23, 233–245 (2012). https://doi.org/10.1007/s00198-011-1546-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1546-9