Abstract
Summary
This paper reviews the evidence for an association between atypical subtrochanteric fractures and long-term bisphosphonate use. Clinical case reports/reviews and case–control studies report this association, but retrospective phase III trial analyses show no increased risk. Bisphosphonate use may be associated with atypical subtrochanteric fractures, but the case is yet unproven.
Introduction
A Working Group of the European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis and the International Osteoporosis Foundation has reviewed the evidence for a causal association between subtrochanteric fractures and long-term treatment with bisphosphonates, with the aim of identifying areas for further research and providing recommendations for physicians.
Methods
A PubMed search of literature from 1994 to May 2010 was performed using key search terms, and articles pertinent to subtrochanteric fractures following bisphosphonate use were analysed.
Results
Several clinical case reports and case reviews report a possible association between atypical fractures at the subtrochanteric region of the femur in bisphosphonate-treated patients. Common features of these ‘atypical’ fractures include prodromal pain, occurrence with minimal/no trauma, a thickened diaphyseal cortex and transverse fracture pattern. Some small case–control studies report the same association, but a large register-based study and retrospective analyses of phase III trials of bisphosphonates do not show an increased risk of subtrochanteric fractures with bisphosphonate use. The number of atypical subtrochanteric fractures in association with bisphosphonates is an estimated one per 1,000 per year. It is recommended that physicians remain vigilant in assessing their patients treated with bisphosphonates for the treatment or prevention of osteoporosis and advise patients of the potential risks.
Conclusions
Bisphosphonate use may be associated with atypical subtrochanteric fractures, but the case is unproven and requires further research. Were the case to be proven, the risk–benefit ratio still remains favourable for use of bisphosphonates to prevent fractures.
Similar content being viewed by others
Introduction
Treatment with bisphosphonates significantly reduces the risk of fractures in men and women with osteoporosis. The evidence is based on high-quality phase III randomized controlled trials (RCTs) with fracture as an endpoint [1–10]. The benefits of bisphosphonates also extend to other disorders of bone metabolism such as glucocorticoid-induced osteoporosis [11], Paget’s disease [12] and bone metastases [13, 14].
Treatment with bisphosphonates is not without adverse effects, but they are generally minor and occur in a minority of patients. The most common adverse effect is gastrointestinal upset with the oral formulations, the frequency of which decreases with intermittent treatment such as once-weekly or monthly regimens. Intravenous (IV) administration of nitrogen-containing bisphosphonates may induce an acute phase reaction which manifests as fever, myalgia and arthralgia, although these side effects usually resolve within a few days of onset [3, 7, 15]. High doses of bisphosphonates given intravenously may impair renal function, and the kidney is a major route of elimination of the bisphosphonates. For this reason, bisphosphonates are not recommended for use in patients with severe renal impairment [16–18]. The use of bisphosphonates has been associated with osteonecrosis of the jaw, but most cases have occurred in patients receiving high-dose IV bisphosphonates for neoplastic bone disease, and osteonecrosis of the jaw has rarely been reported in patients with benign bone diseases [19, 20]. An increased risk of atrial fibrillation has been reported for zoledronic acid [3], but the association may be coincidental [7]. Other uncommon or rare side effects of bisphosphonates include anaemia [21], urticaria [22, 23] and symptomatic hypocalcaemia [22].
In recent years, several clinical case reports and case reviews have reported an association between atypical fractures in patients receiving treatment with bisphosphonates. The majority of these cases have described fractures at the subtrochanteric region of the femur [24–31].
Against this background, the aim of this report was to critically review the evidence for an increased incidence of subtrochanteric fractures after long-term treatment with bisphosphonates, to identify gaps in our knowledge that warrant further research and to provide guidance for healthcare professionals. A PubMed search of literature from 1994 to May 2010 was performed using the search terms ‘bisphosphonate(s)’ AND/OR ‘alendronate’ AND/OR ‘risedronate’ AND/OR ‘ibandronate/ibandronic acid’ AND/OR ‘zoledronate/zoledronic acid’ AND/OR ‘subtrochanter(ic)’ AND ‘fracture’ AND/OR ‘femur/femoral’ AND/OR ‘atypical’ AND/OR ‘low-trauma’ AND/OR ‘low-energy’. Scientific papers pertinent to subtrochanteric fractures following bisphosphonate use were analysed and included in the evidence base.
Characteristics of subtrochanteric fractures
Subtrochanteric fractures have been defined as occurring in a zone extending from the lesser trochanter to 5 cm distal to the lesser trochanter [32]. However, this anatomical classification of subtrochanteric fracture has several variations [33, 34], resulting in variable definitions in published studies [26, 30, 35].
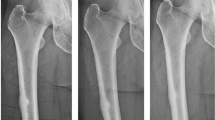
Regardless of the definition used, many case reports and case reviews have suggested that there are several common features of subtrochanteric fractures associated with bisphosphonate use. Major features were that the fractures arose with minimal or no trauma and, on radiography, the fracture line was transverse. Minor features were that fractures were commonly preceded by prodromal pain and, on radiographs, there appeared beaking of the cortex on one side and bilateral thickened diaphyseal cortices [26, 28, 36–39]. This fracture pattern has often been referred to as an ‘atypical subtrochanteric fracture’ [40–42] although, as reviewed below, the distinction between typical and atypical subtrochanteric fractures has not yet been firmly established.
It is worth noting that, on radiography, the appearance of atypical subtrochanteric fractures is similar to that of stress fractures, including a periosteal reaction, linear areas of bone sclerosis and a transverse fracture line. Prodromal pain prior to diagnosis is also common [43]. However, stress fractures are more commonly associated with repeated episodes of increased activity (e.g. participation in sports). Nevertheless, stress fractures due to non-traumatic loading in patients with low bone density do occur (insufficiency fractures) [43]; thus, the terms ‘atypical’ and ‘stress’ are often used interchangeably or in conjunction to describe subtrochanteric fractures in this context. However, this terminology also requires clarification, as not all stress fractures are atypical.
Epidemiology of subtrochanteric fractures
Subtrochanteric fractures are a relatively rare type of hip fracture [44–46], usually resulting from high-energy trauma, pathologic fracture or, in the elderly, low-energy injury involving osteoporotic bone. Several series report the incidence of this fracture [25–28, 30, 36, 37, 47], although the definition of the subtrochanteric site has varied. Nieves et al. reported a large, 11-year epidemiological study of fractures of the hip, subtrochanter, femoral shaft and distal femur in the US population aged ≥50 years using National Hospital Discharge Survey data from the National Center for Health Statistics and MarketScan® (medical claims experience) data [46]. Of all femoral fractures, 3% were at the subtrochanteric region, 5% at the femoral shaft, 5% at the distal femur and 87% were at the proximal femur (i.e. hip). Importantly, this study classified fractures solely according to their location in the femur and did not evaluate the fracture patterns radiographically. Thus, they were not able to determine the incidence of ‘typical’ vs ‘atypical’ subtrochanteric fractures. In men and in women, the incidence rate of each type of fracture remained stable over 5 years but increased exponentially with age (Fig. 1). Each fracture type was more prevalent in women than in men. Seventy-five percent of all femur fracture cases were in women. The mean age at fracture was 80 years old, and those with a subtrochanteric fracture were of a similar age to those with a hip fracture.
Age-specific incidence of femoral fractures according to fracture site in men (X) and women (O) aged ≥50 years (adapted from Nieves et al. [46])
Leung et al. published a retrospective analysis that aimed to document the incidence of low-trauma subtrochanteric or femoral diaphyseal fractures in a Hong Kong hospital over a 5-year period [42]. In all, 88 cases of subtrochanteric fractures and 66 of diaphyseal fractures were identified, accounting for 3.9% and 2.9% of all recorded osteoporotic fractures, respectively.
Thus, although the incidence of subtrochanteric fractures is much lower than other femoral fractures, they are not rare and account for about 3% of all femoral fractures in the elderly. If these estimates were applied to the UK, then more than 2,000 subtrochanteric fractures are expected to occur each year [48], and approximately 48,000 are expected annually worldwide [49].
Subtrochanteric fractures and bisphosphonate exposure
Case reports and case reviews
Twenty-six published case reports and case reviews were identified that were considered relevant, a similar number to that identified in a recent review by Giusti et al. [50].
Concern about bisphosphonate use in relation to atypical subtrochanteric fractures arose from case reports that described patients with subtrochanteric fractures who had been exposed to bisphosphonates, particularly long-term treatment with alendronate (Fosamax®/Fosavance®, alendronate sodium, Merck Sharp & Dohme Limited). The association between long-term bisphosphonate use and unusual diaphyseal fractures was first described by Odvina et al. in 2005 [31] who reported nine patients with osteoporosis or osteopenia who had been treated with alendronate for 3–8 years and sustained atraumatic fractures in the course of their normal daily activities. Three patients had fractures of the femoral shaft and two had fractures of the proximal femur. Of these five patients, fracture healing was radiographically assessed in four. All four patients had delayed or absent fracture healing ranging from 4 months to 2 years while on alendronate treatment.
This and subsequent case reports are summarized in Table 1. The mean and median age of patients was 65 years (range 35–85). All cases involved treatment with alendronate, except for five patients who took risedronate (Actonel®, risedronate sodium, Procter and Gamble Pharmaceuticals) and three who took pamidronate (Aredia®, pamidronate disodium, Novartis Pharmaceuticals Limited). One patient had been taking ibandronate (Bonviva®/Boniva®, ibandronic acid, Roche) for 1 year following long-term alendronate use, and one had been taking risedronate for 5 years following 7 years of pamidronate use. There were no published case reports of subtrochanteric fractures following the use of once-yearly zoledronic acid 5 mg (Aclasta®/Reclast®, zoledronic acid anhydrous, Novartis Pharmaceuticals Limited), although cases following treatment with the monthly 4-mg dose have been reported [36, 38]. The mean and median duration of bisphosphonate use was 7.3 and 7.5 years, respectively (range 1–16), and the majority of patients had unilateral fractures (29 out of 43; 67.4%).
In addition to case reports, several case reviews have been published, which are summarized in Table 2. For example, the characteristics of low-trauma subtrochanteric and diaphyseal fractures were studied retrospectively by Neviaser et al. in all patients admitted to a US trauma centre over a 5-year interval (Table 2) [30]. Radiographs were examined by independent experts to identify fractures with a simple, transverse or short oblique pattern in areas of cortical hypertrophy with a cortical beak. The observers were blinded to patient characteristics, including alendronate use. Seventy patients were identified, of whom 25 were treated with alendronate. Nineteen out of 25 (76%) alendronate-treated patients had the radiographic pattern compared with one out of 45 (2%) non-alendronate-treated patients. Thus, the risk of having an ‘atypical’ subtrochanteric fracture pattern was significantly associated with alendronate use (odds ratio = 139; 95% confidence interval (CI) 19–939; p < 0.0001). The mean duration of treatment with alendronate was 6.2 years (6.9 years in those who had the fracture pattern vs 2.5 years in those who did not) [30]. The authors concluded that there are unique features to bisphosphonate-associated fractures.
Controlled studies
Six studies that utilized control groups were identified that have investigated the association of subtrochanteric fractures with the use of bisphosphonates. In the study of Nieves et al. described above, the rate of subtrochanteric and femoral shaft fractures appeared to be higher than that of other fractures in women taking oral bisphosphonates (Fig. 2) [46], although there is no statistical information provided. It is not known whether excess fractures were due to trauma or not. The study concluded, however, that there was no evidence of an increase in the incidence of subtrochanteric or femoral shaft fracture between 1996 (around the time that bisphosphonates were first introduced) and 2006. Limitations of these data include the lack of radiological and clinical verification and no information on the type of bisphosphonate used or the duration of treatment.
Medical and prescription drug history in US female fracture patients (2002–2006) during the 1 year before index date (adapted from Nieves et al. [46])
In a study by Leung et al., ten patients with subtrochanteric fractures who had received alendronate were identified over a 5-year period. This included one patient who had taken alendronate for 1 year followed by ibandronate for 2 years [42]. The crude incidence of subtrochanteric/femoral diaphyseal fractures associated with prior bisphosphonate use increased over 5 years from 0% in 2003/2004 to 6% in 2004/2005, 8.6% in 2006/2007 and 25% in 2007/2008. This trend was despite a steady annual incidence of subtrochanteric/femoral diaphyseal fractures. It is difficult to draw meaningful conclusions from these data because of the very small sample size (ten subtrochanteric fractures in patients exposed to a bisphosphonate) and the lack of information on bisphosphonate use at other fracture sites. At best, the study documents the increasing use of bisphosphonates over the time of study.
In a small retrospective case–control study, Lenart et al. aimed to identify an association between low-energy subtrochanteric/femoral shaft fractures (according to the Müller AO classification) and long-term bisphosphonate use [29]. Forty-one low-energy subtrochanteric or femoral shaft fracture cases were identified and matched by age, body mass index and race to one low-energy intertrochanteric and femoral neck fracture each.
Fifteen out of the 41 (37%) cases of subtrochanteric or femoral shaft fracture cases were taking bisphosphonates, compared with nine out of 82 (11%) controls (OR = 4.4; 95% CI 1.8–11.4; p = 0.002). Alendronate was the bisphosphonate taken in all cases. Eight out of nine cases in the control group were taking alendronate (one had previously taken etidronate). A radiographic pattern of a simple transverse or oblique fracture, beaking of the cortex on one side and cortical thickening at the fracture site, was observed in ten of the 15 (67%) subtrochanteric/femoral shaft fracture cases taking bisphosphonate and three of the 26 (11%) subtrochanteric/femoral shaft fracture cases not taking bisphosphonate (OR = 15.3; 95% CI = 3.1–76.9; p < 0.001). The duration of bisphosphonate exposure was significantly longer in patients with this X-ray pattern [29].
Koh et al. carried out a retrospective clinical and radiological review of geriatric hip fracture patients at a Singapore tertiary centre over 4 years to assess features that predispose to complete stress fractures [38]. Thirty-two patients with spontaneous or low-energy fractures with metaphyseal–diaphyseal involvement and on bisphosphonate therapy were identified. All were on alendronate therapy except for one who was on monthly zoledronic acid 4 mg and one who had been on risedronate for 6 years following 4 years of alendronate. Of these, 16 patients (median duration of therapy 4.5 years) had radiographic evidence of lateral cortical thickening. Four had cortical stress lesions on the prefracture radiograph (group F) and 12 had cortical stress lesions on the contralateral femur (group C). The type of bisphosphonate taken by patients according to group was not detailed. All patients in group F experienced prodromal thigh discomfort, compared with 25% of patients in group C (p = 0.019), and radiographic evidence of a stress line across the cortical thickening occurred in 100% and 8% of patients, respectively (p = 0.003). At a median follow-up of 23 months, none of the patients in group C had developed a complete fracture. All of these patients except for one had discontinued bisphosphonate therapy; five had not taken any alternative therapy since discontinuation. Nevertheless, eight out of the 11 were asymptomatic, and no new cortical thickening was detected in any of the patients. The authors concluded that, in people taking long-term bisphosphonate therapy, symptomatic cortical stress reactions accompanied by evidence of a stress line across the cortical thickening suggest an increased risk of a complete stress fracture [38].
In the only population-based study that included radiological review of all cases, Schilcher and Aspenberg studied the incidence of stress fractures at the femoral shaft in bisphosphonate-treated patients in four hospitals in Sweden. Women aged over 55 years with fractures of the femoral diaphysis or subtrochanteric region were identified from the operation registry. Preoperative radiographs were examined to identify stress fractures, defined as a transverse fracture of the femoral shaft with cortical thickening. Of 91,956 women identified, 3,087 bisphosphonate users were identified, of whom five had femoral stress fractures. All of these five patients were aged >75 years, and their mean duration of treatment was 5.8 years [66]. Three patients that were not treated with bisphosphonates had stress fractures. All were aged <75 years. The annual incidence of femoral shaft stress fractures in bisphosphonate users was 1/1,000 per year (95% CI 0.3–2) vs 0.02/1,000 (0.004–0.1) per year in control patients. Thus, the risk of such fractures was estimated to be 46 times greater with bisphosphonate use (95% CI 11–200) [65]. An obvious weakness of the study is that, although the confidence intervals were corrected for sample size, the findings were based on just eight femoral shaft stress fractures. The results thus raise a hypothesis to be tested on larger samples.
A larger study is provided by Abrahamsen et al. who studied the epidemiology of subtrochanteric and diaphyseal femur fractures in patients in Denmark treated with alendronate [67]. However, in contrast to the Schilcher and Aspenberg report, in this study, radiographic fracture patterns were not reviewed, and thus, fractures were identified purely based on their location. In patients aged ≥60 years that had subtrochanteric, diaphyseal femur and hip fractures in 2005, the incidence of subtrochanteric (n = 898) and diaphyseal fractures (n = 720) were similar, and the ratio of high-to-low-energy trauma fractures was the same for each of these fracture types (approximately 2.5:1 for each). Exposure to alendronate was also similar between fracture types (approximately 7% each). Patients with subtrochanteric fractures and diaphyseal fractures were more likely to have taken glucocorticoids in the year before fracture than patients with hip fracture (10.9%, 8.4% and 6.5% of patients, respectively).
In a register-based matched cohort analysis, Abrahamsen et al. investigated whether the increase in risk of ‘atypical’ femur fracture in alendronate-treated patients was greater than the increase in risk of ‘typical’ osteoporotic femur fractures (‘typical’ and ‘atypical’ were not defined). In total, 15,187 patients who took alendronate for ≥6 months after the fracture event (the treatment cohort) were compared with two randomly assigned sex-, age- and fracture-matched controls (n = 10,374). The use of alendronate was associated with an increase in the hazard ratio (HR; adjusted for baseline comorbidities) for both subtrochanteric/diaphyseal fractures (HR = 1.46; 95% CI 0.91–2.35; p = 0.12) and hip fracture (HR = 1.45; 95% CI 1.21–1.74; p < 0.001). Subtrochanteric/diaphyseal fractures were equally common in the alendronate-treated (14% of hip fractures) and control patients (13%; p = 0.70). Both hip fractures and subtrochanteric/diaphyseal fractures were significantly lower in patients with higher adherence (HR = 0.47 [0.34–0.65; p < 0.001] and 0.28 [0.12–0.63; p < 0.01], respectively). In a sub-analysis of 178 compliant (medication possession ratio >80%) patients who took alendronate for >6 years, long-term alendronate use was associated with no change in both hip (HR = 1.24 [0.66–2.34]; p = 0.52) and subtrochanteric/diaphyseal fractures (HR = 1.37 [0.22–8.62]; p = 0.74). The incidence of subtrochanteric/diaphyseal fractures was similar in the long-term alendronate (10%) and control (12.5%) groups (10% vs 12.5%, respectively) [67].
This study, in a large number of patients, does not support the hypothesis that exposure to alendronate is associated with an increased frequency of subtrochanteric fractures compared with controls. However, the same study reported that treatment with alendronate was associated with an increased risk of hip fracture. This should not be interpreted as ‘alendronate causes hip fracture’, but only that high-risk patients are exposed to alendronate. The finding also illustrates the difficulties in the interpretation of retrospective observational studies, particularly accounting for selection bias that likely confounds the other much smaller observational studies.
Randomized controlled trials
Black et al. recently reported an analysis of subtrochanteric and diaphyseal fractures in the Fracture Intervention Trial (FIT) of alendronate and its extension [1, 2, 5, 68] and the HORIZON Pivotal Fracture Trial (PFT) of zoledronic acid 5 mg [3]. Twelve fractures in ten patients were documented in the subtrochanteric or diaphyseal region (Table 3) a combined rate of 2.3 per 10,000 patient-years [69]. However, radiographs were not available to confirm typical vs atypical radiographic features. There was no significant increase over placebo in the risk of subtrochanteric/diaphyseal fractures during the FIT, FIT Long-Term Extension (FLEX) or HORIZON-PFT trials. Compared with placebo, the relative hazard was 1.03 (95% CI 0.1–16.5) for alendronate use in the FIT trial, 1.5 (95% CI 0.3–9.0) for zoledronic acid in the HORIZON-PFT and 1.3 (95% CI 0.1–14.7) for continued alendronate use in the FLEX trial. The interpretation of this analysis is limited by the small number of events and the large confidence intervals.
Bilezikian et al. reported the incidence of subtrochanteric fractures in the randomized, placebo-controlled phase III studies of risedronate in post-menopausal osteoporosis, which enrolled more than 15,000 patients. In trials of up to 3 years duration, the mean incidence of subtrochanteric fractures was 0.14% in risedronate 2.5-mg treated patients (n = 4,998), 0.13% in risedronate 5-mg treated patients (n = 5,395) and 0.17% in placebo-treated patients (n = 5,363) [70]. In active control studies of risedronate involving various doses (35 mg once weekly, 75 mg on two consecutive days per month, 150 mg once monthly), no subtrochanteric fractures were reported, and the incidence of hip/femoral fractures was similar to that in the placebo-controlled studies [70].
The manufacturers of ibandronate have assessed their clinical trials database to determine the incidence of subtrochanteric and diaphyseal femoral fractures in women taking ibandronate for post-menopausal osteoporosis. Atypical fractures were defined as ‘mostly non-spine fractures including hip or femur fractures in the subtrochanteric region or shaft and occurring without trauma or in association with low-energy trauma’. For femur fractures, subtrochanteric fracture location was considered as atypical for osteoporosis-related fractures, defined as a region below the lesser trochanter and a junction between the proximal and middle third of the femoral shaft. In the pivotal trials (MF 4380, BONE, MOBILE and DIVA) [4, 71–73], there were nine fracture cases corresponding to these defined locations and characteristics (subtrochanteric, femoral shaft, stress or multiple fractures): six occurred in placebo-treated patients (n = 1,924) and three in ibandronate-treated patients (n = 6,830). In addition, there was one identified case of a femoral shaft fracture in an ibandronate-treated patient in the extension and major phase IIIb trials (MOBILE LTE, DIVA LTE, MOTION and PREVENTION; n = 2,451) [74–77]. Some fractures were reported without identifying the precise location. However, all of these fractures were associated with trauma and thus did not meet the definition for atypical fractures. An additional 5-year analysis of the marketed regimens of ibandronate (150 mg once monthly and 3 mg IV quarterly) was also carried out from the active comparator-controlled trials and their extensions (MOBILE, DIVA, MOTION, MOBILE LTE and DIVA LTE) [71, 72, 74, 75, 77]. No atypical subtrochanteric/diaphyseal femoral fractures were found for either of the marketed regimens (150 mg, n = 1,279; 3 mg, n = 469).
Pharmacovigilance data
Since fractures are the clinical outcome of osteoporosis and no treatments are fully effective, fractures are expected in treated patients. It is likely, however, that the number of reports through pharmacovigilance will be small. The number of postmarketing reports of atypical stress fractures in association with alendronate to circa July 2008 was 115 (of which 84 were femur fractures) and included a large number of the cases reported in the literature [78].
Bilezikian et al. have reported that in more than 10 years of risedronate post-approval surveillance to September 2008 (18 million patient-years of exposure), the reporting rate for subtrochanteric fractures was <0.1 per 100,000 patient treatment years of exposure [70].
Postmarketing data from the manufacturers of zoledronic acid have revealed a similarly low rate of subtrochanteric fractures with zoledronic acid 5 mg. Using the last cutoff date for worldwide Health Authority Reporting prior to January 2010 (Periodic Safety Update Report v6) and assessing all adverse event reports for zoledronic acid 5 mg (579,501 patient-years of exposure), the rate of femoral subtrochanteric fracture reporting was three per 1,000,000 patient treatment years of exposure.
Postmarketing data from the manufacturers of ibandronate have also revealed a low rate of possible atypical fractures occurring in patients receiving ibandronate for the management of postmenopausal osteoporosis. According to their global safety database as of June 2009, cumulative postmarketing exposure of ibandronate yielded a crude reporting rate of possible atypical fractures of approximately one per 1,000,000 patients. Three of the cases involved alendronate treatment followed by ibandronate treatment and were reported in the case series of Ing-Lorenzini et al. [27].
Regulatory perspective
In July 2008, the Pharmacovigilance Working Party (PhVWP) of the Committee for Medicinal Products for Human Use (CHMP) initiated a class review on bisphosphonates and atypical stress fractures. Marketing Authorization Holders supplied information about all preclinical, clinical and future studies, published case reports, postmarketing data, possible mechanisms and proposed risk-minimization activities. Following a PhVWP review of these data in December 2008, the CHMP concluded that there was an association between atypical stress fractures and long-term use of alendronate, due to the distinct fracture pattern, prodromal pain and poor fracture healing. However, the benefit–risk balance of alendronate use was considered favourable. The CHMP highlighted that there was uncertainty concerning a class effect for other bisphosphonates and that switching of bisphosphonates should be avoided at this time. Ultimately, the CHMP recommended that information about atypical stress fractures should be added to the product information for medicinal products containing alendronate [78]. Consequently, the labelling for alendronate (Fosamax®/Fosavance®, Merck Sharp & Dohme Limited) now includes a special warning/precaution for alendronate use, advising discontinuation of bisphosphonate therapy in patients with stress fracture pending evaluation, based on an individual benefit–risk assessment [22, 79]. Alendronate is the only bisphosphonate for osteoporosis treatment that currently carries this warning.
In addition to the 2008 class review, the EMEA released a statement in August 2009 highlighting their 2010 priorities for drug safety research with regards to the long-term adverse skeletal effects of bisphosphonates: (1) generate methodologies to study the link between bisphosphonate use and long-term adverse skeletal events in human populations and (2) measure the incidence of stress/insufficiency fractures in association with high-dose/long-term use of bisphosphonates by class, compound, mode of administration, dose etc. Methods could include meta-analysis or nested case–control studies [80].
In June 2008, the US Food and Drug Administration (FDA) initiated a review of bisphosphonates for a possible association with increased risk of atypical subtrochanteric femur fractures. All available case reports and clinical trial data were requested from all bisphosphonate drug manufacturers and were reviewed alongside the registry data from the large observational study of Abrahamsen et al. [67]. In March 2010, the FDA announced that the data reviewed had not shown a clear connection between bisphosphonate use and the risk of atypical subtrochanteric fractures. Physicians were urged to continue to follow the labelling when prescribing bisphosphonates and patients were instructed not to discontinue their medication unless instructed to do so by their physician [81].
Pathophysiology of subtrochanteric fractures associated with bisphosphonate use
The pathophysiology of atypical low-trauma subtrochanteric fractures following bisphosphonate use is not known. However, preclinical and clinical studies of the effects of bisphosphonates on bone suggest that there are several possible mechanisms that work either alone or in tandem. The organic matrix of the bone determines its toughness, and this matrix is partly made up of bone collagen, which impacts on the bone’s mechanical properties. Bisphosphonate use may negatively affect collagen by preventing or reducing its maturation [82], although this finding has not been consistently replicated [83]. Bisphosphonates may also affect bone mineralization density distribution (BMDD). The more heterogeneous the BMDD, the slower that cracks in the bone will develop and the lower the risk of new cracks and fractures forming [84]. As bisphosphonate treatment reduces bone turnover, the increase in overall mineralization leads to more homogeneous bone—as evidenced by a narrow BMDD [85, 86]—and thus an increased risk of cracks and fractures. Reduced bone turnover also increases the accumulation of microdamage, as cracks are not repaired [87], and reduces bone toughness, which contributes to the increased susceptibility of bone to new cracks [88–90]. Finally, bisphosphonates have differing impacts on different types of fracture. Acute fractures of long bone are not affected by bisphosphonates in the initial healing stages [91–93], as they heal via endochondral ossification. However, stress fractures heal by normal bone remodelling, and thus, bisphosphonates may prevent or delay healing, increasing the likelihood of a complete fracture with little or no trauma. Several reports have reported on bone quality in people with low-trauma fractures taking bisphosphonate therapy.
For example, Odvina et al. reported that cancellous bone histomorphometry in alendronate-treated patients (3–8 years) who sustained spontaneous non-vertebral fractures showed markedly suppressed bone formation, with reduced or absent osteoblastic surface in most patients. Osteoclastic surface was also low in most patients, and eroded surface decreased in half [31]. Odvina et al. reported similar findings in a later report in a comparable patient population [58]. In a case report by Armamento-Villareal et al. of a man who had a low-trauma subtrochanteric fracture after discontinuing 6 years of alendronate treatment, a bone biopsy showed severely decreased trabecular connectivity, a lack of osteoid on trabecular surfaces and an absence of tetracycline labelling [53]. Armamento-Villareal et al. later reported that of 15 bisphosphonate-treated patients (2–10 years; Table 2) who underwent bone biopsies following a low-energy cortical (femoral shaft, pelvis, rib, metatarsal, ankle) fracture, ten had an absence of double-tetracycline label, reduced osteoid surface and thickness suggestive of suppressed trabecular bone remodelling. However, there was no difference in cortical thickness between patients with suppressed (n = 10) and normal (n = 5) turnover [25]. Recent findings by Somford et al., however, suggest an alternative pathophysiology for subtrochanteric fractures associated with bisphosphonate treatment. In a patient who was treated with alendronate for 8 years and subsequently developed spontaneous bilateral subtrochanteric/diaphyseal fractures, biopsies showed a marked decrease in bone formation as expected; however, this was not coupled with the expected decrease in bone resorption. In fact, bone resorption parameters such as osteoclast number were markedly increased in the femur sample. In addition, there was no evidence of hypermineralized bone. This suggests that an imbalance between bone resorption and bone formation at the affected femur—the cause of which is currently unknown—rather than excessive suppression of bone turnover may be the underlying mechanism for subtrochanteric/diaphyseal femoral fractures in bisphosphonate-treated patients [94].
Summary of evidence
The view that bisphosphonates increase the risk of subtrochanteric femoral fractures arises from the case reports and retrospective case reviews that have reported ‘atypical’ subtrochanteric and diaphyseal fractures in patients exposed to bisphosphonates. In all, these data highlight the scope of the problem, i.e. a trend that warrants further investigation. However, the data in their entirety are insufficient proof that long-term bisphosphonate use is the only cause of atypical low-trauma subtrochanteric fractures.
There are several limitations to the existing evidence base: lack of a consensus definition of an atypical subtrochanteric fracture, small numbers of patients involved, lack of radiographs which precludes characterization of the radiographic features of the fractures and incomplete reporting of subject characteristics. In addition, subtrochanteric fractures in general are not atypical fractures; rather, they are part of the natural history of fragility fractures in osteoporosis. They increase in frequency with age in much the same way as does the incidence of other osteoporotic fractures [95]. Although their incidence is much lower than other femoral fractures, they are not rare and account for approximately 3% of all femoral fractures [46]. Thus, the term ‘atypical’ is not synonymous with ‘unexpected’ which is the common interpretation. Rather, the term should be reserved for subtrochanteric fractures that have atypical features, of which some are similar to with those associated with stress.
Therein lies an additional problem in that it has been difficult to provide characteristics of the fracture that are associated with the use of bisphosphonates. Candidate features, which include the prodromal manifestation of incomplete (fissure) fractures, a thickened cortex and a transverse fracture pattern with cortical beaking may be associated with the use of bisphosphonates but, in the absence of blinded evaluation in all cases, may be subject to large observer biases. In addition, in many instances, cases have been complicated, for example, by concomitant exposure to glucocorticoids [25–28, 31, 39, 50, 55, 58, 63, 65], which appears to be a risk factor for subtrochanteric fractures [46].
In terms of evidence-based medicine, the ultimate arbiter for a causal relationship between subtrochanteric fractures and exposure to bisphosphonates might be expected to derive from information from RCTs. All the information available fail to show an association of this fracture with exposure to bisphosphonates, although all RCTs were completed before attention was drawn to the problem, so the documentation of the sites of fracture and any associated features is inevitably incomplete. Furthermore, the frequency of the event is sufficiently low that even large RCTs are insufficiently powered to identify meaningful associations with drug exposure. Finally, the duration of exposure to bisphosphonates may be too short in the setting of RCTs if, as has been suggested, the complication were to increase in frequency with exposure time. Against this background, data from observational studies might be expected to contribute to our understanding, but such studies are fraught with biases and limitations for which it may be difficult to adjust.
Research agenda
The ultimate question for physicians is what type of patient is at the highest risk of an atypical low-trauma subtrochanteric fracture. Thus far, apart from long-term alendronate therapy, suggested risk factors include glucocorticoid, proton-pump inhibitor or calcitonin use and female gender [26, 46, 67]. Thus, a number of urgent issues and areas for research have been identified as follows:
-
1.
Standardized definition of ‘subtrochanteric fracture’, including a definition of ‘atypical’ and ‘typical’ fractures
-
2.
Provision of descriptive epidemiology based on large-scale studies with characterization of radiographic features
-
3.
Definition of fracture incidence by femoral location, mechanism of injury and underlying pathology
-
4.
Identification of risk factors, with greater clarity as to the precise risk factors in patients taking bisphosphonates
-
5.
Pathophysiological studies in relation to risk factors
-
6.
Pathophysiological studies at the tissue level, i.e. is the mechanism of atraumatic (insufficiency) fractures different to that of low-trauma fractures?
-
7.
Long-term, large, prospective, observational studies to assess incidence of subtrochanteric fractures in bisphosphonate-treated vs bisphosphonate-naïve patients. Methods should include (1) futility analysis and (2) radiographic measurements. Outcomes should include (1) adherence, (2) number needed to harm and (3) assessment of temporal relationship between bisphosphonate treatment and fracture type
-
8.
Long-term, large, prospective, observational studies allowing for systematic follow-up of patients with subtrochanteric fractures treated long-term with bisphosphonates, in order to assess fracture healing characteristics (e.g. time to healing, choice of fracture treatment device, adjuvant bone anabolic intervention etc.)
-
9.
Large, prospective, randomized, controlled clinical trials of the efficacy and safety of pharmacological treatment (e.g. strontium ranelate, teriparatide) for patients with subtrochanteric fractures
Conclusions and recommendations
A sense of proportion may be helpful in alleviating the concerns of the medical community. A plausible scenario is that long-term exposure to bisphosphonates (more than 5 years) increases the risk of subtrochanteric femoral fractures twofold. In the UK, using the guidance of the National Osteoporosis Guideline Group, the relative risk of hip fracture is expected to be approximately threefold increased in postmenopausal women identified for treatment [96]. Assuming that the average population risk of hip fracture is 1% per year in postmenopausal women, then 300 hip fractures are expected for every 10,000 patients identified to be at high risk. If these patients were treated and assuming an effectiveness of bisphosphonates of 36% (RR = 0.64) [97], then 108 hip fractures are averted by treatment (and approximately 750 fractures at other sites). On the debit side, three subtrochanteric fractures (both typical and atypical) are to be expected, which might increase to six if bisphosphonates doubled the risk of all subtrochanteric fractures. Under the assumptions of this scenario, the risk–benefit ratio remains very favourable.
Evidence, including that from an EMEA class review, suggests that alendronate use may potentially increase the risk for atypical, low-trauma subtrochanteric fractures, although it is unclear whether this applies to other bisphosphonates. Irrespective of exposure to bisphosphonates, the occurrence of subtrochanteric fractures is an expected finding in patients with osteoporosis. If atypical fractures do occur, then their characteristics are poorly defined, their causality with bisphosphonate exposure insecure and their frequency rare. Bisphosphonates as the cause of atypical fractures at the subtrochanteric site is therefore still merely a hypothesis, though no less important for that. Clearly more research is required from well-designed prospective observational studies, meta-analyses and nested case–control studies.
Thus, the available evidence does not suggest that the well-known benefits of bisphosphonate treatment are outweighed by the risk of these rare, atypical, low-trauma subtrochanteric fractures. Nevertheless, it is recommended that physicians remain vigilant in assessing their patients treated with bisphosphonates for osteoporosis or associated conditions. They should continue to follow the recommendations on the drug label when prescribing bisphosphonates and advise patients of the potential risks. Patients with pain in the hips, thighs or femur should be radiologically assessed and, where a stress fracture is evident, the physician should decide whether bisphosphonate therapy should be discontinued pending a full evaluation, based on an individual benefit–risk assessment. The radiographic changes should be evaluated for orthopaedic intervention—since surgery prior to fracture completion might be advantageous—or be closely monitored.
References
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Chesnut CH III, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, Felsenberg D, Huss H, Gilbride J, Schimmer RC, Delmas PD (2004) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 19:1241–1249
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280:2077–2082
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH III, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
Lyles KW, Colon-Emeric CS, Magaziner JS, Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Hodgson PK, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799–1809
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11:83–91
Sorensen OH, Crawford GM, Mulder H, Hosking DJ, Gennari C, Mellstrom D, Pack S, Wenderoth D, Cooper C, Reginster JY (2003) Long-term efficacy of risedronate: a 5-year placebo-controlled clinical experience. Bone 32:120–126
McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344:333–340
Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, Papanastasiou P, Ferreira A, Hartl F, Fashola T, Mesenbrink P, Sambrook PN (2009) Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 373:1253–1263
Devogelaer JP (2002) Modern therapy for Paget’s disease of bone: focus on bisphosphonates. Treat Endocrinol 1:241–257
Lipton A (2007) Treatment of bone metastases and bone pain with bisphosphonates. Support Cancer Ther 4:92–100
Polascik TJ (2009) Bisphosphonates in oncology: evidence for the prevention of skeletal events in patients with bone metastases. Drug Des Devel Ther 3:27–40
Roche Registration Limited (2009) Bonviva summary of product characteristics. Roche Registration, Hertfordshire
Merck SaDL (2007) Fosamax summary of product characteristics. Merck SaDL, Hertfordshire
Procter & Gamble Pharmaceuticals (2007) Actonel summary of product characteristics. Procter & Gamble Pharmaceuticals, Weybridge
US Food and Drug Administration (FDA) (2009) Drug safety newsletter. Volume 2, Number 2. http://www.fda.gov/Drugs/DrugSafety/DrugSafetyNewsletter/default.htm. Accessed 23 Sep 2010
Bilezikian JP (2006) Osteonecrosis of the jaw—do bisphosphonates pose a risk? N Engl J Med 355:2278–2281
Rizzoli R, Burlet N, Cahall D, Delmas PD, Eriksen EF, Felsenberg D, Grbic J, Jontell M, Landesberg R, Laslop A, Wollenhaupt M, Papapoulos S, Sezer O, Sprafka M, Reginster JY (2008) Osteonecrosis of the jaw and bisphosphonate treatment for osteoporosis. Bone 42:841–847
Novartis Europharm Limited (2009) Aclasta summary of product characteristics. Novartis Europharm, Horsham
Merck Sharp & Dohme Limited (2009) Fosavance summary of product characteristics. Merck Sharp & Dohme, Hertfordshire
Roche Pharmaceuticals (2009) Boniva (ibandronate sodium) injection prescribing information. Roche Pharmaceuticals, Nutley
Guanabens N, Peris P, Monegal A, Pons F, Collado A, Munoz-Gomez J (1994) Lower extremity stress fractures during intermittent cyclical etidronate treatment for osteoporosis. Calcif Tissue Int 54:431–434
Armamento-Villareal R, Napoli N, Diemer K, Watkins M, Civitelli R, Teitelbaum S, Novack D (2009) Bone turnover in bone biopsies of patients with low-energy cortical fractures receiving bisphosphonates: a case series. Calcif Tissue Int 85:37–44
Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS (2007) Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br 89:349–353
Ing-Lorenzini K, Desmeules J, Plachta O, Suva D, Dayer P, Peter R (2009) Low-energy femoral fractures associated with the long-term use of bisphosphonates: a case series from a Swiss university hospital. Drug Saf 32:775–785
Kwek EB, Goh SK, Koh JS, Png MA, Howe TS (2008) An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury 39:224–231
Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, van der Meulen MC, Lorich DG, Lane JM (2009) Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int 20:1353–1362
Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG (2008) Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma 22:346–350
Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY (2005) Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90:1294–1301
Fielding JW, Magliato HJ (1966) Subtrochanteric fractures. Surg Gynecol Obstet 122:555–560
Muller ME, Nazarian S, Koch P, Schatzker J (1990) The AO classification of long bones. http://aofoundation.com/AOFileServer/PortalFiles?FilePath=/Extranet/en/_att/wor/act/fracture_classif/mueller_ao_class.pdf. Accessed 23 Sep 2010
Seinsheimer F (1978) Subtrochanteric fractures of the femur. J Bone Joint Surg Am 60:300–306
Black DM, Genant HK, Bucci-Rechtweg C, Bauer DC, Mesenbrink PG, Palermo L, Nusgarten L, Eastell R (2009) Does zoledronic acid increase risk of atypical subtrochanteric femoral shaft fractures? Results from the HORIZON-PFT. J Bone Miner Res 24 (Suppl 1). http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=918d35dd-6a3d-43f6-b35f-b484a15b81cf. Accessed 23 Sep 2010
Bunning RD, Rentfro RJ, Jelinek JS (2010) Low-energy femoral fractures associated with long-term bisphosphonate use in a rehabilitation setting: a case series. PM&R 2:76–80
Capeci CM, Tejwani NC (2009) Bilateral low-energy simultaneous or sequential femoral fractures in patients on long-term alendronate therapy. J Bone Joint Surg Am 91:2556–2561
Koh JS, Goh SK, Png MA, Kwek EB, Howe TS (2010) Femoral cortical stress lesions in long-term bisphosphonate therapy: a herald of impending fracture? J Orthop Trauma 24:75–81
Visekruna M, Wilson D, McKiernan FE (2008) Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab 93:2948–2952
Kwek EB, Koh JS, Howe TS (2008) More on atypical fractures of the femoral diaphysis. N Engl J Med 359:316–317
Lenart BA, Lorich DG, Lane JM (2008) Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med 358:1304–1306
Leung F, Lau T-W, To M, Luk K-K, Kung A (2010) Atypical femoral diaphyseal and subtrochanteric fractures and their association with bisphosphonates. BMJ Case Reports. doi:10.1136/bcr.10.2008.1073
Brandser EA, Buckwalter JA (1996) Imaging studies for diagnosing stress and insufficiency fractures. Iowa Orthop J 16:70–78
Lunsjo K, Ceder L, Tidermark J, Hamberg P, Larsson BE, Ragnarsson B, Knebel RW, Allvin I, Hjalmars K, Norberg S, Fornander P, Hauggaard A, Stigsson L (1999) Extramedullary fixation of 107 subtrochanteric fractures: a randomized multicenter trial of the Medoff sliding plate versus 3 other screw-plate systems. Acta Orthop Scand 70:459–466
Whitelaw GP, Segal D, Sanzone CF, Ober NS, Hadley N (1990) Unstable intertrochanteric/subtrochanteric fractures of the femur. Clin Orthop Relat Res 252:238–245
Nieves JW, Bilezikian JP, Lane JM, Einhorn TA, Wang Y, Steinbuch M, Cosman F (2010) Fragility fractures of the hip and femur: incidence and patient characteristics. Osteoporos Int 21:299–408
Glennon DA (2009) Subtrochanteric stress fractures in six patients on long term bisphosphonate therapy: a case series. Bone 44:S68–S98
National Institute for Health and Clinical Excellence (2010) Final appraisal determination. Alendronate, etidronate, risedronate, raloxifene and strontium ranelate for the primary prevention of osteoporotic fragility fractures in postmenopausal women (amended). NICE technology appraisal guidance 160 (amended). http://www.nice.org.uk/nicemedia/pdf/TA160guidance.pdf. Accessed 23 Sep 2010
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
Giusti A, Hamdy NA, Papapoulos SE (2010) Atypical fractures of the femur and bisphosphonate therapy. A systematic review of case/case series studies. Bone 47:169–180
Husada G, Libberecht K, Peeters T, Populaire J (2005) Bilateral mid-diaphyseal femoral stress fractures in the elderly. Eur J Trauma 31:68–71
Schneider JP (2006) Should bisphosphonates be continued indefinitely? An unusual fracture in a healthy woman on long-term alendronate. Geriatrics 61:31–33
Armamento-Villareal R, Napoli N, Panwar V, Novack D (2006) Suppressed bone turnover during alendronate therapy for high-turnover osteoporosis. N Engl J Med 355:2048–2050
Cheung RK, Leung KK, Lee KC, Chow TC (2007) Sequential non-traumatic femoral shaft fractures in a patient on long-term alendronate. Hong Kong Med J 13:485–489
Demiralp B, Ilgan S, Ozgur KA, Cicek EI, Yildrim D, Erler K (2007) Bilateral femoral insufficiency fractures treated with inflatable intramedullary nails: a case report. Arch Orthop Trauma Surg 127:597–601
Lee P, van der Wall H, Seibel MJ (2007) Looking beyond low bone mineral density: multiple insufficiency fractures in a woman with post-menopausal osteoporosis on alendronate therapy. J Endocrinol Investig 30:590–597
Sayed-Noor AS, Sjoden GO (2008) Subtrochanteric displaced insufficiency fracture after long-term alendronate therapy—a case report. Acta Orthop 79:565–567
Odvina CV, Levy S, Rao S, Zerwekh JE, Sudhaker RD (2009) Unusual mid-shaft fractures during long term bisphosphonate therapy. Clin Endocrinol (Oxf) 72:161–168
Ali T, Jay RH (2009) Spontaneous femoral shaft fracture after long-term alendronate. Age Ageing 38:625–626
Goddard MS, Reid KR, Johnston JC, Khanuja HS (2009) Atraumatic bilateral femur fracture in long-term bisphosphonate use. Orthopedics 32:607. doi:10.3928/01477447-20090624-27
Sayed-Noor AS, Sjoden GO (2009) Case reports: two femoral insufficiency fractures after long-term alendronate therapy. Clin Orthop Relat Res 467:1921–1926
Cermak K, Shumelinsky F, Alexiou J, Gebhart MJ (2009) Case reports: subtrochanteric femoral stress fractures after prolonged alendronate therapy. Clin Orthop Relat Res 468:1991–1996
Bush LA, Chew FS (2009) Subtrochanteric femoral insufficiency fracture in woman on bisphosphonate therapy for glucocorticoid-induced osteoporosis. Radiol Case Rep 4. doi:1.2484/rcr.v4i1.261
Lee JK (2009) Bilateral atypical femoral diaphyseal fractures in a patient treated with alendronate sodium. Int J Rheum Dis 12:149–154
Edwards MH, McCrae FC, Young-Min SA (2010) Alendronate-related femoral diaphysis fracture—what should be done to predict and prevent subsequent fracture of the contralateral side? Osteoporos Int 21:701–703
Schilcher J, Aspenberg P (2009) Incidence of stress fractures of the femoral shaft in women treated with bisphosphonate. Acta Orthop 80:413–415
Abrahamsen B, Eiken P, Eastell R (2009) Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res 24:1095–1102
Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR (2000) Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 85:4118–4124
Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, de Papp A, Bauer DC (2010) Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med 362:1761–1771
Bilezikian J, Klemes A, Silverman S, Cosman F (2009) Subtrochanteric fracture reports coincident with risedronate use. J Bone Miner Res 24(Suppl 1). http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=0367cfaa-4d0d-47d8-a57a-ff76098839a2. Accessed 23 Sep 2010
Eisman JA, Civitelli R, Adami S, Czerwinski E, Recknor C, Prince R, Reginster JY, Zaidi M, Felsenberg D, Hughes C, Mairon N, Masanauskaite D, Reid DM, Delmas PD, Recker RR (2008) Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol 35:488–497
Miller PD, McClung MR, Macovei L, Stakkestad JA, Luckey M, Bonvoisin B, Reginster JY, Recker RR, Hughes C, Lewiecki EM, Felsenberg D, Delmas PD, Kendler DL, Bolognese MA, Mairon N, Cooper C (2005) Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res 20:1315–1322
Recker R, Stakkestad JA, Chesnut CH III, Christiansen C, Skag A, Hoiseth A, Ettinger M, Mahoney P, Schimmer RC, Delmas PD (2004) Insufficiently dosed intravenous ibandronate injections are associated with suboptimal antifracture efficacy in postmenopausal osteoporosis. Bone 34:890–899
Miller PD, Epstein S, Sedarati F, Reginster JY (2008) Once-monthly oral ibandronate compared with weekly oral alendronate in postmenopausal osteoporosis: results from the head-to-head MOTION study. Curr Med Res Opin 24:207–213
Stakkestad JA, Lakatos P, Lorenc R, Sedarati F, Neate C, Reginster JY (2008) Monthly oral ibandronate is effective and well tolerated after 3 years: the MOBILE long-term extension. Clin Rheumatol 27:955–960
McClung MR, Bolognese MA, Sedarati F, Recker RR, Miller PD (2009) Efficacy and safety of monthly oral ibandronate in the prevention of postmenopausal bone loss. Bone 44:418–422
Bianchi G, Felsenberg D, Czerwinski E, Reid D, Kenwright A, Burdeska A, Recker R (2009) Efficacy of IV ibandronate is maintained over 5 years: the DIVA LTE study. Ann Rheum Dis 68(Suppl 3):494
European Medicines Agency (2009) Assessment report for Fosavance. EMEA/CHMP/188952/2009. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000619/WC500024252.pdf. Accessed 23 Sep 2010
Merck Sharp & Dohme Limited (2010) Fosamax summary of product characteristics. Merck Sharp & Dohme, Hertfordshire
European Medicines Agency (2009) EMEA 2010 priorities for drug safety research. Long-term adverse skeletal effects of bisphosphonates. Doc.Ref: EMEA/493711/2009 Rev.1. European Medicines Agency, London
US Food and Drug Administration (FDA) (2010) FDA drug safety communication: ongoing safety review of oral bisphosphonates and atypical subtrochanteric fractures. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203891.htm. Accessed 23 Sep 2010
Durchschlag E, Paschalis EP, Zoehrer R, Roschger P, Fratzl P, Recker R, Phipps R, Klaushofer K (2006) Bone material properties in trabecular bone from human iliac crest biopsies after 3– and 5–year treatment with risedronate. J Bone Miner Res 21:1581–1590
Boskey AL, Spevak L, Weinstein RS (2009) Spectroscopic markers of bone quality in alendronate treated postmenopausal women. Osteoporos Int 20:793–800
Turner CH, Burr DB (2006) Principles of bone biomechanics. In: Lane NE, Sambrook PN (eds) Osteoporosis and the osteoporosis of rheumatic diseases. Mosby Elsevier, Philadelphia, pp 41–53
Boivin GY, Chavassieux PM, Santora AC, Yates J, Meunier PJ (2000) Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone 27:687–694
Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaushofer K (2001) Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone 29:185–191
Allen MR, Burr DB (2007) Three years of alendronate treatment results in similar levels of vertebral microdamage as after one year of treatment. J Bone Miner Res 22:1759–1765
Allen MR, Iwata K, Phipps R, Burr DB (2006) Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1–year treatment with clinical treatment doses of risedronate or alendronate. Bone 39:872–879
Allen MR, Reinwald S, Burr DB (2008) Alendronate reduces bone toughness of ribs without significantly increasing microdamage accumulation in dogs following 3 years of daily treatment. Calcif Tissue Int 82:354–360
Iwata K, Allen MR, Phipps R, Burr DB (2006) Microcrack initiation occurs more easily in vertebrae from beagles treated with alendronate than with risedronate. Bone 38(Suppl):42
Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, Akiyama T, Shi L, Komatsubara S, Miyamoto K, Norimatsu H (2002) Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res 17:2237–2246
MacDonald MM, Schindeler A, Little DG (2007) Bisphosphonate treatment and fracture repair. BoneKey 4:236–251
Martinez MD, Schmid GJ, McKenzie JA, Ornitz DM, Silva MJ (2010) Healing of non–displaced fractures produced by fatigue loading of the mouse ulna. Bone 46:1604–1612
Somford MP, Draijer FW, Thomassen BJ, Chavassieux PM, Boivin G, Papapoulos SE (2009) Bilateral fractures of the femur diaphysis in a patient with rheumatoid arthritis on long-term treatment with alendronate: clues to the mechanism of increased bone fragility. J Bone Miner Res 24:1736–1740
O’Neill TW (2005) Looking back: developments in our understanding of the occurrence, aetiology and prognosis of osteoporosis over the last 50 years. Rheumatology (Oxford) 44:iv33–iv35
Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A (2008) Case finding for the management of osteoporosis with FRAX—assessment and intervention thresholds for the UK. Osteoporos Int 19:1395–1408
Kanis JA, Borgstrom F, Zethraeus N, Johnell O, Oden A, Jonsson B (2005) Intervention thresholds for osteoporosis in the UK. Bone 36:22–32
Acknowledgements
The Working Group meeting was supported by an unrestricted educational grant from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis. Editorial assistance for the manuscript was provided by Sola Neunie of BioScience Communications, supported by a financial grant from Novartis Pharmaceuticals.
Conflicts of interest
Rene Rizzoli has attended paid advisory boards and received consultancy and lecturing fees from Servier, Novartis, Eli Lilly, Amgen, Roche, Nycomed, Merck Sharp and Dohme and Danone. Kristina Åkesson has received lecturing fees from Medtronics, Novartis, Amgen, Merck and Nycomed. Mary Bouxsein has undertaken consultancy and lecturing commitments for Amgen and Merck & Co. John A. Kanis consults or has received research support from a large number of pharmaceutical companies involved in marketing products for treatment of osteoporosis. He is president of the International Osteoporosis Foundation and serves on its Committee of Scientific Advisors. Nicola Napoli has received grant support from Merck Sharpe and Dohme. Socrates Papapoulos has received consultancy and lecturing fees from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Merck & Co, Novartis, Pfizer and Roche. Jean-Yves Reginster has received consulting fees and attended paid advisory boards for Servier, Novartis, Negma, Lilly, Wyeth, Amgen, GlaxoSmithKline, Roche, Merckle, Nycomed, NPS, Theramex and UCB. He has received invited lecture fees from Merck Sharp and Dohme, Lilly, Rottapharm, IBSA, Genevrier, Novartis, Servier, Roche, GlaxoSmithKline, Teijin, Teva, Ebewee Pharma, Zodiac, Analis, Theramex, Nycomed and Novo Nordisk. He has received grant support from Bristol Myers Squibb, Merck Sharp & Dohme, Rottapharm, Teva, Lilly, Novartis, Roche, GlaxoSmithKline, Amgen and Servier. Cyrus Cooper has undertaken consultancy and lecturing commitments for Alliance for Better Bone Health, Eli Lilly, Novartis, GSK Roche, Servier, MSD and Amgen.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Rizzoli, R., Åkesson, K., Bouxsein, M. et al. Subtrochanteric fractures after long-term treatment with bisphosphonates: a European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int 22, 373–390 (2011). https://doi.org/10.1007/s00198-010-1453-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-010-1453-5