Abstract
Summary
To assess the prescription patterns of anti-osteoporosis medications, three cross-sectional analyses were performed between 2004 and 2006. Women aged 50 and older were identified from the health insurance claims database of the Rhône-Alpes area. HRT prescriptions decreased while bisphosphonates and raloxifene prescriptions increased, respectively, in different age groups.
Introduction
The objective of this study was to assess the prescription patterns of hormone replacement therapy (HRT) and anti-osteoporosis medications (AOM) in post-menopausal French women since the WHI and the revision of the French clinical practice guidelines in 2004.
Methods
Three cross-sectional analyses were performed between 2004 and 2006. Women aged 50 and older who had at least one claim for a prescription for HRT, bisphosphonates or raloxifene were identified from health insurance claims database of the Rhône-Alpes area.
Results
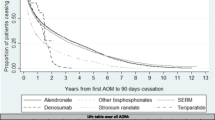
A 39% decrease in the number of women who had HRT was observed (67,241 to 41,024). Twenty-one percent and 18% increases were observed, respectively, for bisphosphonates (39,192 to 47,395) and raloxifene (10,263 to 12,060). HRT and raloxifene were mainly prescribed to women aged 55 to 64 (58% and 39%, respectively), bisphosphonates to women aged 65 to 84 (70%). Ninety-eight percent of women had HRT prescribed by a gynaecologist or a general practitioner (GP). Most AOM were prescribed by a GP; 13% of women had AOM prescribed by a rheumatologist.
Conclusion
Prescriptions for HRT in post-menopausal French women have significantly decreased while bisphosphonates and raloxifene prescriptions have increased, respectively, in different age groups but to a lesser extent than the HRT decrease.


Similar content being viewed by others
References
Manson JE, Bassuk SS, Harman SM et al (2006) Postmenopausal hormone therapy: new questions and the case for new clinical trials. Menopause 13:139–147
Rossouw JE, Anderson GL, Prentice RL et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama 288:321–333
Castelo-Branco C, Palacios S, Calaf J et al (2005) Available medical choices for the management of menopause. Maturitas 52(Suppl 1):S61–S70
Cranney A, Guyatt G, Griffith L et al (2002) Meta-analyses of therapies for postmenopausal osteoporosis. IX: Summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocr Rev 23:570–578
Ettinger B, Black DM, Mitlak BH et al (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. Jama 282:637–645
Barrett-Connor E, Grady D, Sashegyi A et al (2002) Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. Jama 287:847–857
Hodsman AB, Bauer DC, Dempster DW et al (2005) Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 26:688–703
Beral V (2003) Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362:419–427
Agence Nationale d’Accréditation et d’Evaluation en Santé, Agence Française de Sécurité Sanitaire des Produits de Santé. Audition publique: Les traitements hormonaux substitutifs (THS) de la ménopause, Rapport d’orientation 11 mai 2004. Available at: http://agmed.sante.gouv.fr/pdf/10/roths.pdf. Accessibility verified August 16, 2007.
Gayet-Ageron A, Amamra N, Ringa V et al (2005) Estimated numbers of postmenopausal women treated by hormone therapy in France. Maturitas 52:296–305
Morabia A, Costanza MC (2006) Recent reversal of trends in hormone therapy use in a European population. Menopause 13:111–115
Institut National de la Statistique et des Etudes Economiques. Estimation de population par région, sexe et grande classe d’âge - Années 1990 à 2004. Available at: http://www.insee.fr/fr/ffc/docs_ffc/elp_reg_dep.htm. Accessibility verified August 16, 2007
Agence Française de Sécurité Sanitaire des Produits de Santé. Traitement médicamenteux de l’ostéoporose post-ménopausique. Actualisation 2006. Available at: http://agmed.sante.gouv.fr/pdf/5/rbp/ostemarg.pdf. Accessibility verified August 16,2007
Austin PC, Mamdani MM, Tu K et al (2003) Prescriptions for estrogen replacement therapy in Ontario before and after publication of the Women’s Health Initiative Study. Jama 289:3241–3242
Haas JS, Kaplan CP, Gerstenberger EP et al (2004) Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med 140:184–188
Hersh AL, Stefanick ML, Stafford RS (2004) National use of postmenopausal hormone therapy: annual trends and response to recent evidence. Jama 291:47–53
Majumdar SR, Almasi EA, Stafford RS (2004) Promotion and prescribing of hormone therapy after report of harm by the Women’s Health Initiative. Jama 292:1983–1988
Usher C, Teeling M, Bennett K et al (2006) Effect of clinical trial publicity on HRT prescribing in Ireland. Eur J Clin Pharmacol 62:307–310
Caisse Nationale d’Assurance Maladie des Travailleurs Salariés (2004) Ostéoporose: étude des prescriptions des biphosphonates et du raloxifène. Available at: http://www.ameli.fr/fileadmin/user_upload/documents/OSTEOPOROSE.pdf. Accessibility verified August 16, 2007
Black DM, Cummings SR, Karpf DB et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
McClung MR, Geusens P, Miller PD et al (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344:333–340
Rizzoli R (2006) Long-term outcome of weekly bisphosphonates. Clin Orthop Relat Res 443:61–65
Sambrook P, Cooper C (2006) Osteoporosis. Lancet 367:2010–2018
Code de la Sécurité Sociale (2004) Article L.162–4. Journal Officiel du 19 décembre 2003
Perez-Edo L, Ciria Recasens M, Castelo-Branco C et al (2004) Management of osteoporosis in general practice: a cross-sectional survey of primary care practitioners in Spain. Osteoporos Int 15:252–257
Jaglal SB, Weller I, Mamdani M et al (2005) Population trends in BMD testing, treatment, and hip and wrist fracture rates: are the hip fracture projections wrong? J Bone Miner Res 20:898–905
Couris CM, Duclos A, Rabilloud M et al (2007) A seventy percent overestimation of the burden of hip fractures in women aged 85 and over. Bone 41:896–900
Acknowledgements
The authors gratefully acknowledge the contributions of Dr Joëlle Guilhot, Dr Roland Nublat, and Dr Gilbert Lemoine, medical officers at the French Health Insurance of the Rhône-Alpes area.
Conflicts of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huot, L., Couris, C.M., Tainturier, V. et al. Trends in HRT and anti-osteoporosis medication prescribing in a European population after the WHI study. Osteoporos Int 19, 1047–1054 (2008). https://doi.org/10.1007/s00198-008-0587-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-008-0587-1