Abstract
Introduction
A randomized, double-blind, prospective, 24-week clinical trial was performed to evaluate the effects of a combinative agent, Maxmarvil, of calcitriol (0.5 μg) and alendronate (5 mg) on bone metabolism in postmenopausal women.
Methods
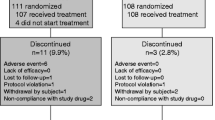
A total of 217 postmenopausal women with osteoporosis were enrolled; 199 patients were randomly assigned to one of two treatment groups (Maxmarvil group or alfacalcidol group). None of the patients were vitamin-D-deficient, as assessed by serum 25-hydroxyvitamin D (25(OH)D), nor had they received any drugs affecting bone metabolism before enrollment. Bone mineral densities (BMD) of L1–L4 and the femur were measured by dual-energy X-ray absorptiometry (DXA) at the initial assessment and after 6 months of treatment. Serum biochemical assays, including serum calcium, 24-h urinary calcium excretion, and bone turnover markers (both bone-specific alkaline phosphatase [bsALP] and urine N-telopeptide [NTx]), were performed at the baseline and after 3 and 6 months of treatment.
Results
In the Maxmarvil group, the BMD of the lumbar spine increased up to 2.42±0.5% from the baseline after 6 months (p<0.05). On the other hand, the change in BMD in the alfacalcidol group was 0.28±0.5% after 6 months. There was no significant difference in femoral BMD between the two groups. The levels of bsALP and NTx were significantly lower in the Maxmarvil group than in the alfacalcidol group (−22.04±3.9% vs. −11.42±2.8% [p<0.05] and −25.46±5.2% vs. 1.24±6.2% [p<0.001], respectively). Interestingly, there was a significantly smaller amount of 24-h urinary calcium in the Maxmarvil group (p<0.05).
Conclusions
Our study demonstrates that a combination of calcitriol and alendronate is quite effective in preventing bone loss, with the advantage of lesser hypercalciuric effect of calcitriol in the postmenopausal osteoporotic women.



Similar content being viewed by others
References
(2001) NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7–29, 2000: highlights of the conference. South Med J 94(6):569–573
Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T (2001) A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab 86(3):1212–1221
Mosekilde L (2005) Vitamin D and the elderly. Clin Endocrinol (Oxf) 62(3):265–281
Lips P, Wiersinga A, van Ginkel FC, Jongen MJ, Netelenbos JC, Hackeng WH, Delmas PD, van der Vijgh WJ (1988) The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab 67(4):644–650
Harinarayan CV (2005) Prevalence of vitamin D insufficiency in postmenopausal south Indian women. Osteoporos Int 16(4):397–402
Richy F, Schacht E, Bruyere O, Ethgen O, Gourlay M, Reginster JY (2005) Vitamin D analogs versus native vitamin D in preventing bone loss and osteoporosis-related fractures: a comparative meta-analysis. Calcif Tissue Int 76(3):176–186
Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, Golub E, Rodan GA (1991) Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 88(6):2095–2105
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348(9041):1535–1541
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280(24):2077–2082
Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350(12):1189–9911
Kushida K, Shiraki M, Nakamura T, Kishimoto H, Morii H, Yamamoto K, Kaneda K, Fukunaga M, Inoue T, Nakashima M, Orimo H (2004) Alendronate reduced vertebral fracture risk in postmenopausal Japanese women with osteoporosis: a 3-year follow-up study. J Bone Miner Metab 22(5):462–468
Iwamoto J, Takeda T, Sato Y, Uzawa M (2005) Early changes in urinary cross-linked N-terminal telopeptides of type I collagen level correlate with 1-year response of lumbar bone mineral density to alendronate in postmenopausal Japanese women with osteoporosis. J Bone Miner Metab 23(3):238–422
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8(9):1137–1148
Monk RD, Bushinsky DA (2003) Kidney stones. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS (eds) Williams textbook of endocrinology. WB Saunders, Philadelphia, Pennsylvania, pp 1411–1425
Shiraki M, Kushida K, Fukunaga M, Kishimoto H, Taga M, Nakamura T, Kaneda K, Minaguchi H, Inoue T, Morii H, Tomita A, Yamamoto K, Nagata Y, Nakashima M, Orimo H (1999) A double-masked multicenter comparative study between alendronate and alfacalcidol in Japanese patients with osteoporosis. The Alendronate Phase III Osteoporosis Treatment Research Group. Osteoporos Int 10(3):183–192
Frediani B, Allegri A, Bisogno S, Marcolongo R (1998) Effects of combined treatment with calcitriol plus alendronate on bone mass and bone turnover in postmenopausal osteoporosis: two years of continuous treatment. Clin Drug Invest 15(3):235–244
Shiraki M, Kushida K, Fukunaga M, Kishimoto H, Kaneda K, Minaguchi H, Inoue T, Tomita A, Nagata Y, Nakashima M, Orimo H (1998) A placebo-controlled, single-blind study to determine the appropriate alendronate dosage in postmenopausal Japanese patients with osteoporosis. The Alendronate Research Group. Endocr J 45(2):191–201
Cummings SR, Karpf DB, Harris F, Genant HK, Ensrud K, LaCroix AZ, Black DM (2002) Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 112(4):281–289
Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD (2003) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 18(6):1051–1056
Hochberg MC, Ross PD, Black D, Cummings SR, Genant HK, Nevitt MC, Barrett-Connor E, Musliner T, Thompson D (1999) Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Fracture Intervention Trial Research Group. Arthritis Rheum 42(6):1246–1254
Greenspan SL, Holland S, Maitland-Ramsey L, Poku M, Freeman A, Yuan W, Kher U, Gertz B (1996) Alendronate stimulation of nocturnal parathyroid hormone secretion: a mechanism to explain the continued improvement in bone mineral density accompanying alendronate therapy. Proc Assoc Am Physicians 108(3):230–238
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rhee, Y., Kang, M., Min, Y. et al. Effects of a combined alendronate and calcitriol agent (Maxmarvil®) on bone metabolism in Korean postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Osteoporos Int 17, 1801–1807 (2006). https://doi.org/10.1007/s00198-006-0200-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0200-4