Abstract
Introduction
Although intermittent parathyroid hormone (PTH) injection can lead to strong anabolic effects on bone, daily subcutaneous injection is a disadvantage for patient acceptance. We have developed a nasal spray formula of parathyroid peptide [hPTH(1–34)] with peak serum hPTH(1–34) concentrations by nasal spray of 1,000 μg similar to those by subcutaneous injections of 20 μg hPTH(1–34).
Methods
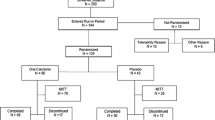
To determine the clinical efficacy and safety of nasal hPTH(1–34) spray, a randomized, open-labeled clinical trial was conducted in subjects with osteoporosis. Ninety osteoporotic subjects age 52–84 years (mean 66.5 years) were randomly assigned to receive either 250 μg (PTH250, n=31), 500 μg (PTH500, n=30), or 1,000 μg (PTH1000, n=29) of daily nasal hPTH(1–34) spray for 3 months. All received daily supplements of 300 mg calcium and 200 IU vitamin D3.
Results
Daily nasal hPTH(1–34) spray for 3 months increased lumbar bone mineral density (L-BMD) in a dose-dependent manner, and the PTH1000 group showed a 2.4% increase in L-BMD from baseline. Only the 1,000-μg dose produced consistent and statistically significant changes in markers of bone turnover; after 3 months, median serum type I procollagen N-propeptide (PINP) and osteocalcin increased 14.8% and 19.4% from baseline, while urinary type I collagen N-telopeptide (NTX) decreased 16.4%. Seven subjects developed transient hypercalcemia at 3 h after nasal hPTH(1–34) spray, but none of the subjects developed sustained hypercalcemia.
Conclusion
These observations demonstrate that nasal hPTH(1–34) spray is safe and well tolerated and can rapidly increase L-BMD. The results warrant further studies to examine its long-term efficacy on bone mass and fractures.

Similar content being viewed by others
References
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Hodsman AB, Hanley DA, Ettinger MP, Bolognese MA, Fox J, Metcalfe AJ, Lindsay R (2003) Efficacy and safety of human parathyroid hormone-(1–84) in increasing bone mineral density in postmenopausal osteoporosis. J Clin Endocrinol Metab 88:5212–5220
Miki T, Nakatsuka K, Naka H, Masaki H, Imanishi Y, Ito M, Inaba M, Morii H, Nishizawa Y (2004) Effect and safety of intermittent weekly administration of human parathyroid hormone 1–34 in patients with primary osteoporosis evaluated by histomorphometry and microstructural analysis of iliac trabecular bone before and after 1 year of treatment. J Bone Mineral Metab 22:569–576
Orimo H, Sugioka Y, Fukunaga M et al (1998) Diagnostic criteria of primary osteoporosis. J Bone Miner Metab 16:139–150
Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Oh-Hashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H (2001) Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab 19:331–337
Kohno T, Murasugi N, Sakurai H, Watabe K, Nakamuta H, Koida M, Sugie Y, Nomura M, Yanagawa A (1998) Development of a highly sensitive and specific two-site enzyme immunoassay for parathyroid hormone (1–34): application to pharmacokinetic study on intranasal parathyroid hormone (1–34) in human. J Clin Lab Anal 12:268–275
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) study group. Osteoporos Int 11:83–91
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant K, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture intervention trial research group. Lancet 348:1535–1541
Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert, Ensrud KE, Avioli LV, Lips P, Cummings SR (1999) Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA 282:637–645
Marcus R, Wang O, Satterwhite J, Mitlak B (2003) The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevalent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner Res 18:18–23
Chen P, Satterwhite JH, Licata AA, Lewiecki EM, Sipos AA, Misurski DM, Wagman RB (2005) Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res 20:962–970
Ettinger B, San Martin J, Crans, Gerald, Crans G, Pavo I (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19:745–751
Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215
Acknowledgements
The following investigators participated in the trial: Shiro Murakoshi, Sapporo Orthopaedic and Circulatory Hospital; Kimiyoshi Tsuga, Sapporo Maruyama Orthopaedic Hospital; Tomoko Hasunuma, The Kitasato Institute Bioiatric Center; Hikaru Ishii, Shin-nihonbashi Ishii Clinic; Masanari Omata, Oimachi Orthopaedic Clinic; Noriyuki Fujita, Komonji Hospital; Keizo Ohmori, Fukuoka Wajiro Hospital; and Hiroo Yamane, Toyooka Dai-Ichi Hospital. The authors thank Dr. Paul Langman for his assistance with English usage. This work was funded by Chugai Pharmaceutical Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Matsumoto, T., Shiraki, M., Hagino, H. et al. Daily nasal spray of hPTH(1–34) for 3 months increases bone mass in osteoporotic subjects: a pilot study. Osteoporos Int 17, 1532–1538 (2006). https://doi.org/10.1007/s00198-006-0159-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0159-1