Abstract
Background
Several pitfalls arise in the assessment of postmortem blood alcohol concentrations (BAC). The purpose of the present study was to evaluate in a controlled and systematic manner in a porcine model whether a postmortem congener pattern can provide evidence for antemortem or postmortem ethanol neogenesis.
Methods
Ethanol was administered intravenously to six pigs, whereas six control pigs remained sober. The animals were euthanized 1h after the start of administration, and peripheral and heart blood (HB) as well as muscle tissue were collected. The animals were stored at room temperature and the aforementioned range of samples was collected daily for 3 days. Samples were analyzed for ethanol and congener substances by headspace gas chromatography-mass spectrometry.
Results
Over the course of storage, ethanol formation was observed in the sober pigs, resulting in a median BAC of 0.24 g/kg body weight. The BAC in the pigs with alcohol remained comparatively stable. In addition, a distinct increase in n‑propanol, n‑butanol, and acetaldehyde was observed. The median blood concentration of n‑propanol in sober animals was higher after storage than that of pigs with alcohol, but no significant differences could be substantiated between the two groups (p > 0.05). Acetaldehyde and n‑butanol concentrations in HB of the sober pigs increased to the level of the pigs with alcohol at death after 3 days. Until the end of the experiment (3 days postmortem), no significant differences in concentrations were detected. Concentrations in muscle tissue did not increase to the same extent.
Discussion
In the present study, no marker could be identified that could reliably discriminate antemortem ethanol ingestion from postmortem genesis.
Zusammenfassung
Hintergrund
Bei der Beurteilung postmortaler Blutalkoholkonzentrationen (BAK) ergeben sich mehrere Fallstricke. In der vorliegenden Studie sollte im Schweinemodell kontrolliert und systematisch evaluiert werden, ob ein postmortales Begleitstoffmuster Anhaltspunkte für eine ante mortem oder post mortem erfolgte Ethanolgenese liefern kann.
Methodik
Sechs Schweinen wurde Ethanol intravenös verabreicht, während 6 Kontrollschweine nüchtern blieben. Eine Stunde nach Applikationsbeginn wurden die Tiere getötet und peripheres und zentrales Blut sowie Muskelgewebe entnommen. Die Kadaver wurden bei Raumtemperatur gelagert und das genannte Probenspektrum jeweils täglich über 3 Tage entnommen. Die Proben wurden mittels Headspace-Gaschromatographie-Massenspektrometrie auf Ethanol und Begleitstoffe untersucht.
Ergebnisse
Über den Verlauf der Lagerung wurde eine Ethanolneubildung in den nüchternen Schweinen beobachtet, die zu einer medianen BAK von 0,24 g/kg Körpergewicht führte. Die BAK in den alkoholisierten Schweinen blieb vergleichsweise stabil. Zudem konnte ein deutlicher Anstieg an n‑Propanol, n‑Butanol und Acetaldehyd beobachtet werden. Die mediane Blutkonzentration von n‑Propanol lag bei ursprünglich nüchternen Tieren nach Lagerung über der der alkoholisierten Schweine, es ergaben sich jedoch keine signifikanten Unterschiede (p > 0,05). Die Acetaldehyd- und n‑Butanol-Konzentrationen im Herzblut der nüchternen Schweine stiegen innerhalb vom 3 Tagen auf das Niveau der bei Todeseintritt alkoholisierten Schweine an. Bis zum Versuchsende (3 Tage post mortem) konnten keine signifikanten Unterschiede in den Konzentrationen mehr festgestellt werden. Die Konzentrationen im Muskelgewebe stiegen nicht in demselben Maße an.
Diskussion
In der vorliegenden Studie konnte kein Marker ermittelt werden, mit dem sich eine ante mortem erfolgte Ethanolaufnahme von einer postmortalen Genese zuverlässig diskriminieren ließe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ethanol still represents one of the most widely used psychoactive substances worldwide [1] and is relevant in daily forensic medicine practice with respect to road traffic investigations, criminal offences and fatalities, including intoxications [2]. Detection of the substance is relatively simple. For this purpose, Widmark, alcohol dehydrogenase method or headspace (HS) gas chromatography methods have been established decades ago. Interpreting the analytical results of postmortem specimens can be more difficult because ethanol can be newly formed or also degraded by microorganisms after death [3, 4]. To overcome this problem, various approaches have been discussed in the literature, such as the analysis of several matrices besides blood [3, 5, 6]. An additional tool could also be the simultaneous detection of so-called congeners, which are also contained in alcoholic beverages and are used, for example, to verify or falsify so-called hip flask defense claims. Some of the substances are also found as decomposition alcohols, in some cases in significantly higher concentrations than after antemortem consumption of ethanolic beverages [7]. Therefore, it was assumed that concentrations of putrefaction alcohols change in a similar manner as ethanol that originated from postmortem neogenesis; however, no reliable marker suited for an easy distinction has so far been established. Moreover, a decrease of the ethanol concentration in specimens of individuals with alcohol at the time of death was discussed [7]. Regarding congeners, it is questionable, whether an antemortem alcoholisation affects the postmortem concentration time course; however, most results are based on case reports with type and amount of ethanol ingestion being rarely precisely known. Applying a sophisticated pig model, the present study aimed to clarify in a controlled and systematic manner, whether it is possible to differentiate between antemortem sober and animals with alcohol based on a congener substance pattern.
Experimental
Materials
-
Ethanol absolute (Fisher Scientific, Loughborough, UK)
-
Methanol, 1‑butanol, ethylmethylketone, isopropanol, isobutanol, n‑propanol, 2‑butanol, tertiary-butanol (for analysis); methylacetate, propylacetate, propionaldehyde, acetaldehyde, isobutyraldehyde, 2‑methyl-1-butanol (for synthesis), sodium sulfate anhydrous for analysis (Merck, Darmstadt, Germany)
-
3‑methyl-1-butanol, isovaleraldehyde (Sigma-Aldrich, Steinheim, Germany)
-
Sodium chloride 0.9% for infusion (B Braun, Melsungen, Germany)
-
Poire Williams Reserve (Eckerts Wacholder Brennerei GmbH, Tholey, Germany)
-
T 61 euthanizing agent (Intervet Deutschland GmbH, Unterschleißheim, Germany)
-
Agilent (Waldbronn, Germany) gas chromatography (GC) Oven 8890 equipped with a DB-624 column (30 m x 250 µm, 1.4 µm); mass spectrometer (MS) 5977B with ultra-inert extractor source; HS sampler 7697A
-
Mass Hunter Workstation for quantitative analysis Version 10.0 (Agilent)
-
Graphpad Prism 9.4.1 (GraphPad Software, San Diego, CA, USA)
Study design
The animals used for the study were domestic male pigs (Swabian Hall strain, n = 12) with a body weight (BW) of 38.2–50.8 kg. Surgical procedures have already been described elsewhere [8,9,10]. The animals were divided into two groups. Animals in group 1 (n = 6) received no alcohol before death, pigs in group 2 (n = 6) were given alcohol (0.7 g ethanol per kg BW via a central venous catheter) 0.5–1 h before death. For this purpose, a solution containing 25% of the ethanolic beverage (Poire Williams Reserve) and 75% of 0.9% sodium chloride solution was prepared. To assure complete distribution of the alcoholic beverage, a latency time of another 30 min followed before drawing of the peripheral blood (PB) sample. Afterwards, the pigs of both groups (with alcohol and sober) were killed using T61 (0.12 mL/kg BW). Immediately after death, the thoracic cavity was opened and a heart blood (HB) specimen was collected. The thoracic cavity was closed again and samples were taken from the pigs’ psoas muscle. Finally, the pigs were stored at room temperature (RT) in a supine position and PB from the brachial or femoral veins, HB and muscle samples from the hind leg were repeatedly taken 1, 2 and 3 days (postmortem interval, PMI 1–3) after death.
Sample preparation
In a 10 mL HS vial, about 750 mg of sodium sulfate, 450 µL of demineralized water, 500 µL IS solution (tert-butanol, 10 mg/L) and 50 mg of blood or muscle were mixed and stored for about 1 h at RT. For calibrators and quality control samples, 50 µL of water were replaced by the respective stock solution. Then, the samples were put into the autosampler of the HS-GC-MS system. After analysis, the vials were opened and dried for at least 12 h at 105 °C. The weight of the vials was measured before and after analysis. From the difference between the aqueous and dry weight, the percentage of water was estimated.
Analytical method
The aforementioned set-up was used for analysis. The GC oven settings were as follows: 40 °C for 3.5 min, increased to 60 °C in 4 min and increased to 90 °C in 1 min (kept for 3 min, total run time: 11.5 min). The transfer line to the MS had a temperature of 260 °C, the MS source was heated to 230 °C, MS quad to 130 °C. Electron energy was set to 70 eV. The MS was run in selected ion monitoring mode with a dwell time of 50 ms per ion. HS vials were incubated in the HS oven for 15 min at 70 °C. Helium was used as carry gas with a constant flow at 0.9 mL/min. The ion masses collected during the different time segments over the run are shown in Table 1. The method was validated for whole blood according to the guidelines of the Society of Toxicological and Forensic Chemistry (GTFCh) [11] and other international guidelines [12]. The calibration ranges, lower limits of quantification (LLOQ) as well as limits of detection (LOD) are depicted in Table 1. Relatively high concentration ranges were chosen, thus the method would not be suitable for a routine analysis of congeners, e.g., to verify hip flask drink claims.
Statistical evaluation
To assess a significant change during the postmortem storage time of the animals as well as to compare between sober and animals with alcohol, different statistical tests were performed. A non-parametric Friedmann test followed by a Dunn’s multiple comparison test were carried out to check for time-dependent changes in concentrations. For comparison of the two groups, a non-parametric Mann-Whitney U test was applied.
Concentration of ethanol and congeners in the administered Poire Williams Reserve
Analysis of the applied liquor in different dilutions revealed ethanol concentrations being mostly in accordance with the labelled 40% ethanol (v/v). The ethanol and congener concentrations are listed in Table 2.
Results
The perimortem concentrations (mean and median) in samples from both groups are depicted in Table 3. As every specimen was tested negative for 2‑butanol, 2‑methyl-1-butanol and 3‑methyl-1-butanol, those analytes were omitted hereafter. In the animals that remained sober before death, no ethanol was found in the examined samples. Only small amounts of acetone and isopropanol below or near the LLOQ were found in some animals. The remaining analytes were tested negative. In the animals with alcohol, ethanol concentrations of nearly 1 g/L were found in all matrices. N‑propanol could also be found in blood and muscle at similar levels. The concentrations of acetone and n‑butanol were highest in HB and lowest in muscle. Acetaldehyde was detected in all blood samples, the median concentration in HB was higher than in PB. In muscle, acetaldehyde could be detected in a concentration below the LLOQ in only one animal. The methanol concentrations were approximately the same in the different matrices. Isopropanol and isobutanol were detected in relatively low amounts under the LLOQ in most samples (Table 3). Only in HB specimens of two pigs, were very high concentrations of isopropanol detected. Those can be considered as outliers.
Postmortem concentration changes
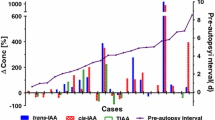
The postmortem concentrations (mean and median) at PMI 3 in samples from both groups are depicted in Table 4. The median concentration changes and significant changes (p < 0.05) of ethanol, acetaldehyde, n‑propanol and n‑butanol from PMI 1–3 compared to PMI 0 are shown in Fig. 1.
Sober pigs
Ethanol was not detected until PMI 2 in PB (n = 3), HB (n = 4), and muscle tissue (n = 1). At PMI 3 ethanol was detectable in PB samples of 4 animals, HB samples of 6 animals, and muscle tissue samples of 3 animals. High interindividual concentration differences could be observed.
At PMI 1, n‑butanol was detected in PB of 1 and HB of 5 animals. At PMI 2, it was found in PB specimens of 2 pigs, in HB specimens of 3 pigs and in muscle specimens of 1 animal. At PMI 3, n‑butanol was quantified in PB of 3, HB of 6 and in muscle tissue of 3 pigs. Regarding the course of median concentrations (Fig. 1), in HB samples a continuous increase was determined, which was most pronounced from PMI 2 to PMI 3. This increase was only significant (p < 0.05) comparing PMI 0 to PMI 3.
N‑propanol was detected from PMI 2 in PB of 3 animals, HB of 4 animals and muscle tissue of 1 animal. At PMI 3, the substance was detectable in PB of 4 animals and in HB and muscle tissue of 6 animals. From PMI 2 to PMI 3, a considerable increase of median concentrations in PB and HB was observed with concentrations in HB changing most.
Acetaldehyde was detected in PB of 2 pigs at PMI 2 and PMI 3, therefore no median concentration changes could be observed. The analyte was first detected in HB of 4 pigs at PMI 2 and showed the most significant increase of concentrations from PMI 2 to PMI 3. In muscle tissue, only one pig had a detectable concentration at PMI 3.
Methanol was only detected in PB of one pig at PMI 3 and in HB of the same pig at PMI 2 and 3. In muscle, increasing concentrations were found in 2 animals at PMI 1 and in 4 animals at PMI 2 and 3.
Concerning acetone and isopropanol, only minor changes of the concentrations were observed postmortem.
Regarding isobutanol, this analyte was detected in PB of 3 pigs only at PMI 2 and in another pig only at PMI 3. In HB, it was only detected in 2 pigs starting at PMI 1 with increasing concentrations until PMI 3.
Pigs with alcohol
Ethanol was detected in every sample analyzed with only small changes from PMI 0 to PMI 3 in HB and muscle samples (Fig. 1). In PB, a significant decrease (p < 0.05) was detected between PMI 0 and PMI 2 and PMI 3.
N‑butanol was detected in almost every specimen, except for muscle tissue (n = 4). In this tissue, median concentrations remained relatively stable over time (Fig. 1). In PB, they remained almost stable until PMI 2. An increase from PMI 2 to PMI 3 could be observed. Looking at the median n‑butanol concentrations in HB, a slight decrease over time was observed compared to PMI 0 (Fig. 1).
N‑propanol was also detected in each analyzed sample. The mean concentrations remained almost stable from PMI 0 to PMI 2 and increased distinctly from PMI 2 to PMI 3 in PB and HB (Fig. 1). The change in mean concentrations in PB was statistically significant from PMI 0 to PMI 2 as well as PMI 3.
Acetaldehyde could be determined in every analyzed blood sample. Regarding PB, median concentrations remained stable until PMI 1 and increased over the next 2 days. In HB, a slight median increase from PMI 0 to PMI 1 but no significant (p > 0.05) changes could be observed over the course of the 3 PMI. In muscle tissue, 2 individuals showed detectable concentrations at PMI 1 and PMI 2. At PMI 3, 5 pigs had detectable acetaldehyde concentrations in muscle tissue; however, the concentrations stayed far below the measured blood concentrations.
Methanol was found in every analyzed postmortem specimen. The median concentrations of methanol slightly decreased from PMI 0 to PMI 3 in PB. In HB and muscle tissue, the concentrations increased to a small extent.
Isopropanol and isobutanol were detected in every postmortem sample with median concentrations staying almost stable.
Discussion
General remarks
The concentrations found in PB were compared to the theoretically calculated expected values resulting from the administered amount of beverage using the Widmark formula or Bonte formulae [13] (30 min after the end of drinking, see Table 2), although the formulae were developed empirically during drinking experiments in humans and therefore might not be fully applicable to the porcine model. Nevertheless, the predicted blood concentrations did not differ considerably as compared to the determined concentrations, as shown in Table 3. Thus, the anatomic similarities render pigs to be a suitable model for studies on toxicokinetics of alcohol in man. The elevated n‑butanol concentrations in HB at the time of death might indicate that the distribution was not yet completed; however, it is unclear why this analyte was detected in the animals with alcohol, although only low amounts were present in the administered beverage.
Comparison between sober pigs and pigs with alcohol
As expected, comparison of the perimortem samples led to significant differences between sober pigs and pigs with alcohol. This was the case for all types of samples regarding ethanol, n‑propanol, n‑butanol as well as acetaldehyde, and can be attributed to the ethanol administration before death. After 24 h (PMI 1), the difference between the two groups was not significant for n‑butanol in HB. After 48 h a significant difference could be observed between the values of n‑propanol in muscle as well as ethanol in all samples. After 72 h (PMI 3) only ethanol values were found to be significantly different in all samples. Comparison between sober pigs and pigs with alcohol for n‑propanol and n‑butanol did not lead to significant differences.
Postmortem, a relatively moderate increase of ethanol was observed in the initially sober animals, which can be attributed to putrefaction processes. Theanimals with alcohol also showed a slight median increase of the HB concentration, but in total not as distinct as in the sober group. These findings may be explained by a possible additional degradation of ethanol by microorganisms due to initial availability in the matrices, as postulated by Kästner [7]. In contrast, muscle tissue showed a median lower increase compared to blood in the sober animals, which could indicate a comparatively higher isolation from microbial infestation.
In some previous studies, attempts were made to distinguish antemortem uptake from postmortem neogenesis, for example, by introducing cut-off values or concentration ratios.
Nanikawa et al. [14] suggested that an antemortem ethanol concentration could be validly estimated based on the concentration ratios of ethanol to n‑propanol (ethanol/propanol). They found maximum ratios of less than 10 times for muscle and less than 20 times for blood in sober animals. This conclusion could not be further substantiated by our results as after 3 days of storage, ratios of up to 85 were found in blood and in 1 pig a ratio of about 200 was found in muscle tissue caused by relatively low n‑propanol concentrations contrary to the findings of Nanikawa et al. [14]. These discrepancies might have been expected as the thesis has already been challenged over the last decades in several studies, e.g., in a recent systematic study in rats conducted by Liang et al. [15]. They compared the respective concentrations of ethanol and n‑propanol as well as their ratio in rats with and without an antemortem ethanol intake (2 g/kg via gavage) after a storage at RT for 2 days or a refrigeration for 4 days [15]. In alignment to our results, no correlation was found between ethanol and n‑propanol concentrations in either sober animals or animals with alcohol [15].
The threshold value of 1.04 mg/L for n‑propanol postulated by Boumba et al. [16] to indicate a postmortem neoformation, was actually exceeded in most cases only from PMI 2. Correspondingly, the n‑propanol concentration was below the threshold value in no sample in which postmortem produced ethanol was detected, supporting the aforementioned thesis. Nevertheless, an excessive consumption of a congener-rich beverage might easily lead to antemortem n‑propanol concentrations exceeding the threshold, leading to false positive hints at a postmortem neoformation; however, the associated high BAC might identify such extreme cases effortlessly.
High postmortem acetaldehyde concentrations were already suggested as a marker to assess an antemortem ethanol intake [17]. Chen et al. compared ethanol, n‑propanol, and acetaldehyde concentrations in ethanol-positive blood samples of living persons, well-preserved and heavily putrefied deceased people. Furthermore, an in vitro study covering several days was conducted with initially alcohol negative and alcohol positive blood samples of living and dead people. The threshold value of 140 mg/L acetaldehyde in blood proposed by Chen et al. [17] as a hint at postmortem neoformation of ethanol was not exceeded in the pig blood samples; however, this discrepancy might be attributed to a less pronounced putrefaction in the current study.
Limitations of the study
It has to be taken into particular consideration that the samples examined are non-human. Although the pig body displays many similarities with the human organism in terms of anatomy and metabolism, a difference in the amount and composition of microorganisms cannot be ruled out and may exert a considerable influence on the results. In addition, the experimental setting must also be taken into account. Due to a simultaneously conducted pharmacokinetic study, the pig bodies were dissected postmortem, furthermore resealed and reopened several times after death for sampling. This procedure carries the risk of additional microbial contamination of the organism by surrounding organs, the environment or instruments used. As a result, putrefaction processes could be altered as compared to a body that remained completely untouched over the storage period. Another limitation could be the limited storage time associated with a comparably mild extent of putrefaction of the examined cadavers. Thus, a study that would cover a longer PMI might be better suited to mirror the conditions in a heavily decomposed cadaver; however, even under the present conditions, the sampling of PB was relatively difficult. Finally, it should be mentioned that in the present study only storage at RT was investigated. Yet, storage at other temperatures or ambient conditions could lead to different results.
Conclusion
Based on the obtained results, no congener alcohol could be identified as specific marker to distinguish between antemortem ethanol uptake and postmortem neogenesis. In agreement with previously published results, regarding routine cases, an evaluation of postmortem blood alcohol findings and the integration of analytical results from multiple matrices, biomarkers and the overall circumstances are recommended. Considering the relatively constant concentration of some congeners in the postmortem matrices, high postmortem levels might indicate a previous consumption of (congener-rich) alcoholic beverages.
References
World Health Organization (2019) Global status report on alcohol and health 2018
Sjögren H, Eriksson A, Ahlm K (2000) Role of alcohol in unnatural deaths: a study of all deaths in Sweden. Alcohol Clin Exp Res 24:1050–1056
Kugelberg FC, Jones AW (2007) Interpreting results of ethanol analysis in postmortem specimens: a review of the literature. Forensic Sci Int 165:10–29
Ziavrou K, Boumba VA, Vougiouklakis TG (2005) Insights into the origin of postmortem ethanol. Int J Toxicol 24:69–77
Pajunen T, Vuori E, Lunetta P (2018) Epidemiology of alcohol-related unintentional drowning: is post-mortem ethanol production a real challenge? Inj Epidemiol 5:39
Savini F, Tartaglia A, Coccia L et al (2020) Ethanol determination in post-mortem samples: correlation between blood and vitreous humor concentration. Molecules 25:2724
Kästner J (2010) Ethanol und Fäulnisalkohole in Blut, Urin und Muskel in verschiedenen Stadien der Leichenfäulnis. https://refubium.fu-berlin.de/bitstream/handle/fub188/13886/Kaestner_Jana_Dissertation.pdf. Accessed 5 June 2023
Doerr AA, Nordmeier F, Walle N et al (2020) Can a recently developed pig model be used for in vivo metabolism studies of 7‑azaindole-derived synthetic cannabinoids? A study using 5F-MDMB-P7AICA. J Anal Toxicol 45(6):593–604
Nordmeier F, Sihinevich I, Doerr AA et al (2021) Toxicokinetics of U‑47700, tramadol, and their main metabolites in pigs following intravenous administration: is a multiple species allometric scaling approach useful for the extrapolation of toxicokinetic parameters to humans? Arch Toxicol 95:3681–3693
Schaefer N, Kroll AK, Korbel C et al (2019) Distribution of the (synthetic) cannabinoids JWH-210, RCS‑4, as well as 9‑tetrahydrocannabinol following pulmonary administration to pigs. Arch Toxicol 93:2211–2218
Peters FT, Hartung M, Herbold M et al (2009) Anhang B zur Richtlinie der GTFCh zur Qualitätssicherung bei forensisch-toxikologischen Untersuchungen Anforderungen an die Validierung von Analysenmethoden. Toxichem Krimtech 76:185–208
Peters FT, Drummer OH, Musshoff F (2007) Validation of new methods. Forensic Sci Int 165:216–224
Bonte W (1987) Begleitstoffe alkoholischer Getränke. Max Schmidt-Römhild, Lübeck
Nanikawa R, Ameno K, Hashimoto Y et al (1982) Medicolegal studies on alcohol detected in dead bodies—alcohol levels in skeletal muscle. Forensic Sci Int 20:133–140
Liang H, Kuang S, Guo L et al (2016) Assessment of the role played by n‑propanol found in postmortem blood in the discrimination between antemortem consumption and postmortem formation of ethanol using rats. J Forensic Sci 61:122–126
Boumba V, Kourkoumelis N, Ziavrou K et al (2019) Estimating a reliable cutoff point of 1‑propanol in postmortem blood as marker of microbial ethanol production. J Forensic Sci Med 5:141–146
Chen X, Dong X, Zhu R et al (2020) Abnormally high blood acetaldehyde concentrations suggest a potential of postmortem ethanol generation. J Anal Toxicol 45:748–755
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A.A. Doerr, F. Nordmeier, N. Walle, M.W. Laschke, M.D. Menger, M.R. Meyer, P.H. Schmidt and N. Schaefer declare that they have no competing interests.
The experiment was performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the German legislation on protection of animals (permission number 32/2018).
Additional information
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.

Scan QR code & read article online
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Doerr, A.A., Nordmeier, F., Walle, N. et al. Drunk or just putrefied?. Rechtsmedizin 33, 471–478 (2023). https://doi.org/10.1007/s00194-023-00653-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00194-023-00653-w