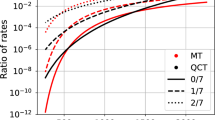
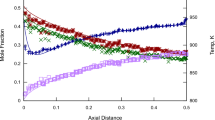
Abstract
The effect of employing four different reactor models, Zeldovich–von Neumann–Döring (ZND), constant volume (CV), constant pressure (CP), and the Shock routine from Chemkin, to perform detonation-relevant chemical modeling was assessed. The simulation results were compared in terms of characteristic length scales and chemical analyses with four representative mixtures: \(\hbox {H}_{2}{-}\hbox {O}_{2}{-}\hbox {N}_{2}\), \(\hbox {H}_{2}{-}\hbox {NO}_{2}/{\hbox {N}_{2}}{\hbox {O}_{4}}\), \({\hbox {C}_{3}}{\hbox {H}_{8}}{-}\hbox {O}_{2}{-}\hbox {N}_{2}\), and dimethyl ether (DME)–\(\hbox {O}_{2}{-}\hbox {CO}_{2}\). The following conclusions were drawn: (i) CV and CP reactor models shorten the induction zone length and strengthen the energy release rate in most mixtures. In terms of chemical kinetics, the impact of CP and CV reactor models is quite limited for \(\hbox {H}_{2}{-}\hbox {O}_{2}{-}\hbox {N}_{2}\) and \(\hbox {H}_{2}{-}\hbox {NO}_{2}/{\hbox {N}_{2}}{\hbox {O}_{4}}\) mixtures. However, the C2 branch is enhanced in CV and CP reactor models for \({\hbox {C}_{3}}{\hbox {H}_{8}}{-}\hbox {O}_{2}{-}\hbox {N}_{2}\) mixture. Moreover, both reactor models weaken the intermediate-temperature chemistry and promote the high-temperature chemistry for DME–\(\hbox {O}_{2}{-}\hbox {CO}_{2}\) mixtures; (ii) the Shock module can be employed to perform detonation modeling, as it provided similar results to the ZND simulations for all investigated mixtures; and (iii) the ZND reactor model is preferred over the zero-dimensional reactor models, while the Shock module of ANSYS is equivalent to the ZND reactor.









Similar content being viewed by others
References
Wolański, P.: Detonative propulsion. Proc. Combust. Inst. 34(1), 125–158 (2013). https://doi.org/10.1016/j.proci.2012.10.005
Wolański, P.: Application of the continuous rotating detonation to gas turbine. Appl. Mech. Mater. 782, 3–12 (2015). https://doi.org/10.4028/www.scientific.net/AMM.782.3
Li, J.-M., Teo, C.J., Khoo, B.C. , Wang, J.-P., Wang, C.: Detonation Control for Propulsion. Springer International Publishing, Cham (2018). https://doi.org/10.1007/978-3-319-68906-7
Wang, Y., Qi, Y., Xiang, S., Mével, R., Wang, Z.: Shock wave and flame front induced detonation in a rapid compression machine. Shock Waves 28(5), 1109–1116 (2018). https://doi.org/10.1007/s00193-018-0832-2
Masri, A.R.: Challenges for turbulent combustion. Proc. Combust. Inst. 38(1), 121–155 (2021). https://doi.org/10.1016/j.proci.2020.07.144
IEA, World Energy Outlook 2020, Paris (2020). https://www.iea.org/reports/world-energy-outlook-2020
Lee, J.H.S.: Dynamic parameters of gaseous detonations. Annu. Rev. Fluid Mech. 16, 311–336 (1984). https://doi.org/10.1146/annurev.fl.16.010184.001523
Lee, J.H.S.: The Detonation Phenomenon. Cambridge University Press, Cambridge (2008). https://doi.org/10.1017/CBO9780511754708
Ng, H.D., Ju, Y., Lee, J.H.S.: Assessment of detonation hazards in high-pressure hydrogen storage from chemical sensitivity analysis. Int. J. Hydrog. Energy 32, 93–99 (2007). https://doi.org/10.1016/j.ijhydene.2006.03.012
Presles, H.N., Desbordes, D., Guirard, M., Guerraud, C.: Gaseous nitromethane and nitromethane-oxygen mixtures: a new detonation structure. Shock Waves 6, 111–114 (1996). https://doi.org/10.1007/BF02515194
Sturtzer, M.-O., Lamoureux, N., Matignon, C., Desbordes, D., Presles, H.-N.: On the origin of the double cellular structure of the detonation in gaseous nitromethane and its mixtures with oxygen. Shock Waves 14, 45–51 (2005). https://doi.org/10.1007/s00193-004-0236-3
Joubert, F., Desbordes, D., Presles, H.-N.: Detonation cellular structure in \({\text{ NO }}_{2}/{\text{ N }}_{2}{\text{ O }}_{4}\)-fuel gaseous mixtures. Combust. Flame 152(4), 482–495 (2008). https://doi.org/10.1016/j.combustflame.2007.11.005
Virot, F., Khasainov, B., Desbordes, D., Presles, H.-N.: Two-cell detonation: losses effects on cellular structure and propagation in rich \(\text{ H}_{2}\)–\({\text{ NO}_{2}}/{{\text{ N}_{2}}{\text{ O}_{4}}}\)–Ar mixtures. Shock Waves 20(6), 457–465 (2010). https://doi.org/10.1007/s00193-010-0283-x
Eckett, C.A., Quirk, J.J., Shepherd, J.E.: The role of unsteadiness in direct initiation of gaseous detonations. J. Fluid Mech. 421, 147–183 (2000). https://doi.org/10.1017/S0022112000001555
Davidenko, D., Mével, R., Dupré, G.: Numerical study of the detonation structure in rich \(\text{ H}_{2}\)–\({\text{ NO }}_{2}/{\text{ N }}_{2}{\text{ O }}_{4}\) and very lean \(\text{ H}_{2}\)–\({\text{ N}_{2}}\text{ O }\) mixtures. Shock Waves 21(2), 85–99 (2011). https://doi.org/10.1007/s00193-011-0297-z
Mével, R., Gallier, S.: Structure of detonation propagating in lean and rich dimethyl ether–oxygen mixtures. Shock Waves 28(5), 955–966 (2018). https://doi.org/10.1007/s00193-018-0837-x
Mével, R., Melguizo-Gavilanes, J., Radulescu, M.: ZND structure of cool detonation in dimethyl ether–oxygen–carbon dioxide mixtures. Asia-Pacific Conference on Combustion, Sydney, Paper 415 (2017)
Liang, W., Mével, R., Law, C.K.: Role of low-temperature chemistry in detonation of n-heptane/oxygen mixtures. Combust. Flame 193, 463–470 (2018). https://doi.org/10.1016/j.combustflame.2018.03.035
Mével, R.: Etude de mécanismes cinétiques et des propriétés explosives des systèmes hydrogène-protoxyde d’azote et silane-protoxyde d’azote application à la sécurité industrielle. PhD Thesis, Université d’Orléans (2009)
Eude, Y.: Développement d’un outil de simulation numérique des écoulements réactifs sur maillage auto-adaptatif et son application à un moteur à détonation continue. PhD Thesis, Université d’Orléans (2011)
Gaillard, T.: Etude numérique du fonctionnement d’un moteur à détonation rotative. PhD Thesis, Université Paris-Saclay (2017)
Mével, R., Davidenko, D., Austin, J.M., Pintgen, F., Shepherd, J.E.: Application of a laser induced fluorescence model to the numerical simulation of detonation waves in hydrogen–oxygen–diluent mixtures. Int. J. Hydrog. Energy 39(11), 6044–6060 (2014). https://doi.org/10.1016/j.ijhydene.2014.01.182
Chatelain, K.P., Mével, R., Melguizo-Gavilanes, J., Chinnayya, A., Xu, S., Lacoste, D.A.: Effect of incident laser sheet orientation on the OH-PLIF imaging of detonations. Shock Waves 30, 689–702 (2020). https://doi.org/10.1007/s00193-020-00963-y
Rojas Chavez, S.B., Chatelain, K.P., Guiberti, T.F., Mével, R., Lacoste, D.A.: Effect of the excitation line on hydroxyl radical imaging by laser induced fluorescence in hydrogen detonations. Combust. Flame 229, 11399 (2021). https://doi.org/10.1016/j.combustflame.2021.111399
Browne, S., Zeigler, J., Shepherd,J.E.: Numerical solution methods for shock and detonation jump conditions. Technical Report FM2006-006, Graduate Aeronautical Laboratories California Institute of Technology (2008)
Goodwin D., Moffat H., Speth R.: Cantera: an object-oriented software toolkit for chemical kinetics, thermodynamics, and transport processes. http://www.cantera.org (2015), version 2.2.0
Shepherd, J.E.: Chemical kinetics of hydrogen–air–diluent detonations. Prog. Astronaut. Aeronaut. 106, 263–293 (1986). https://doi.org/10.2514/5.9781600865800.0263.0293
ANSYS: Academic research mechanical, release 19.4
Chatelain, K., Mével, R., Menon, S., Blanquart, G., Shepherd, J.E.: Ignition and chemical kinetics of acrolein–oxygen–argon mixtures behind reflected shock waves. Fuel 135, 498–508 (2014). https://doi.org/10.1016/j.fuel.2014.07.004
ANSYS Chemkin theory manual 17.0 (15151), Reaction Design, San Diego (2015)
He, Y.Z., Mével, R.: Effect of hydroxyl radical precursor addition on LTC-affected detonation in DME-\(\text{ O}_2\)–\(\text{ CO}_2\) mixtures. Shock Waves 30(7), 789–798 (2020). https://doi.org/10.1007/s00193-020-00974-9
Han, W., Huang, J., Liang, W., Wang, C., Mével, R., Law, C.K.: Unsteady propagation of detonation with multi-stage heat release. Fuel 296, 120666 (2021). https://doi.org/10.1016/j.fuel.2021.120666
Dorofeev, S.B., Sidorov, V.P., Dvoinishnikov, A.E.: Deflagration to detonation transition in large confined volume of lean hydrogen–air mixtures. Combust. Flame 104, 95–110 (1996). https://doi.org/10.1016/0010-2180(95)00113-1
Mével, R., Javoy, S., Lafosse, F., Chaumeix, N., Dupré, G., Paillard, C.-E.: Hydrogen-nitrous oxide delay times: shock tube experimental study and kinetic modelling. Proc. Combust. Inst. 32(1), 359–366 (2009). https://doi.org/10.1016/j.proci.2008.06.171
Mével, R., Chatelain, K., Blanquart, G., Shepherd, J.E.: An updated reaction model for the high-temperature pyrolysis and oxidation of acetaldehyde. Fuel 217, 226–239 (2018). https://doi.org/10.1016/j.fuel.2017.12.060
Bhagatwala, A., Luo, Z., Shen, H., Sutton, J.A., Lu, T., Chen, J.H.: Numerical and experimental investigation of turbulent DME jet flames. Proc. Combust. Inst. 35(2), 1157–1166 (2015). https://doi.org/10.1016/j.proci.2014.05.147
Mével, R., He, Y.: Effect of oxygen atom precursors addition on LTC-affected detonation in DME-\(\text{ O}_{2}\)–\(\text{ CO}_{2}\) mixtures. Shock Waves 30, 799–807 (2020). https://doi.org/10.1007/s00193-020-00953-0
Mével, R., Davidenko, D., Lafosse, F., Chaumeix, N., Dupré, G., Paillard, C.-É., Shepherd, J.E.: Detonation in hydrogen–nitrous oxide–diluent mixtures: an experimental and numerical study. Combust. Flame 162(5), 1638–1649 (2015). https://doi.org/10.1016/j.combustflame.2014.11.026
Gallier, S., Le Palud, F., Pintgen, F., Mével, R., Shepherd, J.E.: Detonation wave diffraction in \(\text{ H}_{2}\)–\(\text{ O}_{2}\)–Ar mixtures. Proc. Combust. Inst. 36(2), 2781–2789 (2017). https://doi.org/10.1016/j.proci.2016.06.090
Mével, R., Lafosse, F., Catoire, L., Chaumeix, N., Dupré, G., Paillard, C.-E.: Induction delay times and detonation cell size prediction of hydrogen–nitrous oxide–diluent mixtures. Combust. Sci. Technol. 180, 1858–1875 (2008). https://doi.org/10.1080/00102200802261340
Ng, H.D., Chao, J., Yatsufusa, T., Lee, J.H.S.: Measurement and chemical kinetic prediction of detonation sensitivity and cellular structure characteristics in dimethyl ether–oxygen mixtures. Fuel 88, 124–131 (2009). https://doi.org/10.1016/j.fuel.2008.07.029
Chatelain, K.P., Mével, R., Lacoste, D.A.: Correction of reaction models using collision limit violation analyses: application to a silane reaction model. Combust. Flames 217, 346–359 (2020). https://doi.org/10.1016/j.combustflame.2020.03.028
Shepherd, J.E.: Ignition modeling and the critical decay rate concept. GALCIT Report EDL2019-002, California Institute of Technology (2020). https://shepherd.caltech.edu/EDL/publications/reprints/cdr_modelEDL2019-002.pdf
Acknowledgements
This work was funded by the King Abdullah University of Science and Technology through the baseline funding (BAS/1/1396-01-01). The ANSYS simulations were performed in KAUST. Yizhuo He was funded by China Postdoctoral Science Foundation (Grant Number 2019M650674).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by G. Ciccarelli.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Governing equations
Appendix: Governing equations
The present appendix describes how the CV, CP, and ZND reactor models can be established from first principles. This enables to clearly understand the differences between these three types of reactors, which are all adiabatic. From the first law of thermodynamics [43], \(\delta Q = \delta E + \delta W\), where Q, E, and W are the heat, the internal energy, and the work, respectively. As \(\delta Q =0\), we obtain
where e is the specific internal energy; P is the pressure; and v is the specific volume. Considering gaseous species which obey the perfect gas law, the total internal energy of a mixture is given by
where k is the total number of chemical species and \(Y_i\) and \(e_{i}\) are the mass fraction and the internal energy of species i, respectively. From the internal energy definition (i.e., \(\text {d}e = \text {c}_{v} \text {d}T\), where T is the temperature) and (3), the specific heat capacity at constant volume (\(c_{v}\)) of a gas mixture is obtained by
Let us recall the perfect gas law
where \(\tilde{R}\) is the universal gas constant divided by the molecular weight (\(\tilde{R}=R{/}W\)), as well as the mass fraction equation
where t is the time; \(\dot{\omega _{i}}\) is the chemical source term; and \(\rho \) is the density. Substituting (3)–(6) into (2), one obtains
If the first law of thermodynamics is expressed as a function of enthalpy (h)
(7) can be expressed as
where \(c_{P}\) is the specific heat capacity at constant pressure.
Considering a constant volume process, (7) reduces to
where as considering a constant pressure process, (9) reduces to
The constant volume reactor model corresponds to (10) and (6), whereas the constant pressure reactor model corresponds to (11) and (6).
To establish the ZND model, let us recall the Euler equations
where \(\nabla \) is the nabla operator; u is the flow velocity; \(\dot{\omega _{i}}^{\prime }=\dot{\omega _{i}}/W\); and \(e_\mathrm{{t}}\) is the specific total energy and is given by
Since the ZND model considers a steady planar shock wave followed by a reaction zone, (12), (13), and (15) reduce to
The energy equation (14) is replaced by the adiabatic change equation [14] given by
where c is the speed of sound and \(\dot{\sigma }\) is the thermicity. Substituting (17) and (18) into (20) and using the definition of the Mach number, \(M=\frac{u}{c}\), we obtain
Equations (21) to (23) correspond to the ZND model expressed as a function of distance. For numerically solving this system of equation, it is convenient to employ a Lagrangian description. Recall that for a steady process
where \(\text {D}\)/\(\text {D}t\) is the Lagrangian derivative. The ZND model becomes
where \(\eta \) is the sonic parameter given by \(\eta =1 - M^{2}\).
To enable further comparison with the CV and CP reactor models, it is useful to establish the temperature-gradient equation along the path of a Lagrangian particle for the ZND model. Taking the substantial derivative of (5) leads to
Substituting (27) and (28) into (30) and using (29) as well as the definition of the mixture molecular weight, \(W_{\text {mix}}=1/\sum _{i}^{k}(Y_i/W_i)\), we obtain
Rights and permissions
About this article
Cite this article
Chatelain, K.P., He, Y., Mével, R. et al. Effect of the reactor model on steady detonation modeling. Shock Waves 31, 323–335 (2021). https://doi.org/10.1007/s00193-021-01022-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00193-021-01022-w