Abstract
Introduction and Hypothesis
Despite the high prevalence of fecal incontinence, existing treatment options may be inadequate. Drugs that enhance the tone of the anal sphincter complex could potentially be an effective pharmacological approach. This study investigated the effect of the tricyclic antidepressant imipramine on anal sphincter tone in healthy women, employing anal acoustic reflectometry as the evaluating method.
Methods
In a double-blind, randomized, placebo-controlled crossover study, 16 healthy female volunteers were randomized to one of two treatment sequences. The participants attended two study visits separated by at least 7 days’ washout. At each visit, they received a single dose of 50 mg imipramine or matching placebo, in alternating order. We assessed the anal opening pressure under the resting state and during voluntary squeezing of the pelvic floor. Measurements were performed pre-dose and 1 h after drug administration, corresponding to the estimated time of peak plasma concentration of imipramine.
Results
All participants completed the study. In total, 44% of the participants reported at least one adverse effect, primarily anticholinergic. Compared with placebo, imipramine increased anal opening pressure by 15.2 cmH2O (95% confidence interval [CI] 2.0–28.2 cmH2O, p = 0.03) in the resting state and 15.1 (95% CI 4.2–26.0 cmH2O, p = 0.01) cmH2O during squeezing.
Conclusions
The findings indicate that imipramine increases anal sphincter tone in healthy women. However, further research is required to evaluate its clinical impact on individuals with fecal incontinence. This research also demonstrates the effectiveness of using anal acoustic reflectometry for assessing pharmacological effects on anal sphincter function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fecal incontinence (FI) is a prevalent, debilitating health care problem with a significant impact on the affected individuals’ quality of life [1, 2]. Despite the high prevalence of FI, treatment options remain limited [3, 4].
Off-label administration of low-dose tricyclic antidepressants (TCAs) has been explored for managing idiopathic FI [5]. However, evidence for this treatment approach is scarce. An open-label, uncontrolled study with 18 FI patients reported that a 4-week regimen of low-dose amitriptyline improved incontinence scores, reduced the frequency of rectal motor complexes, and improved the rectal-to-anal pressure ratio during these rectal motor complexes compared with baseline [6]. Although these suggested benefits of amitriptyline have not been confirmed in a double-blind, placebo-controlled setting, theoretical arguments exist for the possible effect of amitriptyline and other TCAs on anorectal continence function. TCAs may increase external anal sphincter tone by elevating serotonin and noradrenaline levels, i.e., neurotransmitters known to stimulate anal sphincter motor neuron activity [7]. Yet, assessment of anal sphincter function and evaluation of pharmacological treatments targeting sphincter dysfunction are complex challenges. The evaluation predominantly relies on conventional anorectal manometry, a method widely acknowledged to be the standard investigational approach. However, this technique is encumbered by considerable variability in its measurements. Such inconsistency hampers its utility, particularly in distinguishing between individuals with FI and those without [8].
Urethral pressure reflectometry (UPR), a measurement technique originating from urogynecology that utilizes acoustic reflectometry, offers high sensitivity and reproducibility compared with conventional catheter-based methods [9, 10]. With its demonstrated utility in the dynamic assessment of urethral sphincter function and in assessing drug efficacy for stress urinary incontinence, UPR has paved the way for the adaptation of acoustic reflectometry in anal sphincter assessment, now known as anal acoustic reflectometry (AAR) [11]. AAR has shown potential in providing reliable physiological measurements of anal sphincter function, displaying reproducibility comparable with anorectal manometry as well as demonstrating correlation with FI symptom severity [12,13,14].
In this paper, we evaluated the impact of imipramine on anal opening pressure (AOP) using the AAR technique, hypothesizing that imipramine would increase AOP compared with placebo. A secondary objective was to estimate outcome variability for AOP during the resting state and squeezing, facilitating sample size calculations for future trials of potential pharmacological treatments for FI.
Materials and Methods
In this study conducted at Zelo Phase 1 Unit at Copenhagen University Hospital - Bispebjerg and Frederiksberg, Copenhagen, Denmark, we investigated the effect of a single dose of imipramine (50 mg) on urethral and anal pressure in healthy women. The findings from the urethral pressure measurements and a thorough description of the study design, detailed eligibility criteria, and methodologies have been published by Kornholt et al. [15].
The study was approved by the Regional Ethics Committee of the Capital Region of Denmark (approval number H‐17007330) and the Danish Medicines Agency (EudraCT no. 2017‐000119‐18), and was registered at www.ClinicalTrials.org (identifier NCT03102645). Further, the study adhered to the Declaration of Helsinki and Good Clinical Practice guidelines. All participants provided written informed consent upon enrolment.
Study Design
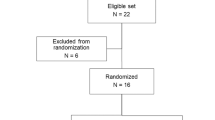
This study was designed as a double-blind, randomized, placebo-controlled crossover study in healthy women. We recruited 16 healthy female volunteers by advertisement and randomized them to one of two treatment sequences (imipramine to placebo or placebo to imipramine). The Capital Region Pharmacy performed the balanced randomization (50% of the participants receiving imipramine at the first study visit) and prepared the numbered blinded dosing kits containing 50 mg imipramine and a visually identical placebo according to the sequence allocation list. The participants were consecutively assigned randomization numbers specifying their dosing kit. Imipramine was selected as the TCA study drug for this combined UPR and AAR study owing to its notable off-label use in managing stress urinary incontinence [16].
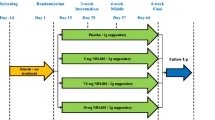
On each of the two study visits, separated by at least 7 days of washout, baseline measurements of the urethral and anal pressure were performed using UPR and AAR respectively. Subsequently, a single dose of 50 mg imipramine or placebo was administered to the participants. After 1 h of rest, corresponding to the mean time to peak plasma concentration for imipramine, 1-h post-dose UPR and AAR measurements were performed. The participants were interviewed about adverse events at the end of study visits 1 and 2, the start of study visit 2, and via telephone 5 days following the final study visit.
Eligibility Criteria
Healthy women aged 18 to 55 with a body mass index (BMI) between 18.5 and 30.0 kg/m2, who were nonsmokers and consented not to breastfeed, become pregnant, and participate in other clinical trials during the study period, were eligible for this study. Primary exclusion criteria were pregnancy, any clinically relevant history or evidence of disease evaluated by the investigator, history of clinically significant urinary incontinence, and the use of any prescription or over-the-counter drugs in the 2 weeks before study drug administration (except for paracetamol and hormonal contraceptives) [15].
Anal Acoustic Reflectometry Outcome Assessment
The AAR assessments and the parameters obtained from these have previously been described [17,18,19]. The AAR measurement catheter consists of a 45-cm polyvinyl chloride (PVC) tube with a thin, fully collapsible, polyurethane bag, 70 mm long, mounted on the distal end, which can be inflated to a maximum diameter of 5 mm. At its proximal end, the PVC tube is connected to a probe with a loudspeaker, a microphone, and another PVC tube to which a handheld syringe, used to inflate and deflate the bag, is connected. Sound waves initiated by a digital signal processor are amplified by the loudspeaker, reflected from the bag, and recorded by the microphone. By applying the Ware–Aki algorithm to the reflected sound waves, computer software (Oticon A/S Rhinometrics 5.6.1.1, Copenhagen, Denmark) calculates the cross-sectional area of every millimeter along the 70-mm-long bag and, in this way, corresponding pressure measurements and cross-sectional areas are obtained. In the further analysis, only the measurements from the point of minimal measurable cross-sectional area, representing the high-pressure zone of the anal canal, are used.
With the participant in the lithotomy position, the polyurethane bag is placed in the anal canal using a baby feeding tube as a guidewire. AAR measurements are recorded during rest and during voluntary contractions of the pelvic floor (squeeze). During the resting condition, the pressure is increased from 0 to 200 cmH2O over 7 s and decreased again to 0 cmH2O over 7 s. This cycle (inflation/deflation) is then repeated ten times, and the mean value of these ten measurements is used for analysis. Subsequently, the participant is asked to contract the pelvic floor (“as trying not to pass gas”). When familiar with the correct squeezing technique, the pressure is increased from 0 to 200 cmH2O in 7 s, and while the participant relaxes the pelvic floor, the pressure is decreased again. This squeezing procedure is repeated five times, and the mean value of five measurements is used for analysis. The total time to obtain a complete set of AAR measurements is approximately 4 min.
All UPR and AAR examinations were performed by a single operator (TR), whereas the AOP was calculated and agreed upon by two of the authors (TR and NK). All involved were blinded to the participants’ sequence allocation until statistical analyses were completed, implicating analysis of the treatment effect as A versus B before we requested the complete unblinding of the study from the hospital pharmacy.
Sample Size Calculations and Statistical Analysis
Based on a prior AAR study, we anticipated a within-subject standard deviation (SD) of 21 cmH2O for the squeezing opening pressure [11]. With a sample size of 16 subjects, our study had a power of 80% to detect a 15 cmH2O difference in squeezing opening pressure between imipramine and placebo using a two-sided significance level of 0.05.
The baseline demographics of the subjects and the within-subject standard deviation (SD) were summarized descriptively. Changes in mean AOP (imipramine versus placebo) from baseline to the 1-h outcome were analyzed using cros analyses, which adjust for period effects. As generally recommended, we ensured a long washout between the study visits to minimize the risk of carry-over effects and omitted formal tests for carry-over [20, 21]. To investigate potential placebo effects, changes in mean AOP from baseline to the 1-h outcome on placebo days were analyzed using paired t tests. Safety data were summarized descriptively.
The statistical analyses for this paper were performed using RStudio version 1.0.136 (RStudio, Boston, MA, USA). Graphical plots were generated using GraphPad Prism version 9.4.1 for Windows (GraphPad Software, San Diego, CA, USA).
Results
A total of 16 women were screened and enrolled in the study, and all of them successfully completed the study protocol. The median age among the participants was 25.5 (range 20–55) years, and median body mass index was 22.8 (range 20.4–27.6) kg/m2.
Mean AOP (mean of the ten measurements at rest and the five measurements during squeezing) per participant, pre-dose and in response to placebo and imipramine, are shown in Fig. 1. Compared with placebo, imipramine increased the anal opening pressure from baseline to 1-h post-dose with 15.2 cmH2O (95% confidence interval [CI] 2.0–28.2 cmH2O, p = 0.03) in the resting state and 15.1 cmH2O (95% CI 4.2–26.0 cmH2O, p = 0.01) during squeezing. Table 1 displays the estimated within-subject standard deviations.
The mean change (SD) from baseline to 1-h post-dose on the placebo days was −3.0 (16.4) cmH2O in the resting state and −1.4 (11.8) cmH2O during squeezing. These changes were not statistically significant when analyzed using paired t tests (p = 0.5 and 0.6 respectively).
During two study visits, 7 participants experienced 14 adverse events. One of these was reported on the placebo day (stomach cramps), the remaining after imipramine administration. The most frequent adverse events during imipramine treatment were drowsiness/sedation (5), dizziness (3), and dry mouth (3). There were no adverse events associated with the AAR procedure.
Discussion
In this randomized, double-blind, placebo-controlled crossover study involving 16 healthy women, a single dose of 50 mg imipramine increased AOP statistically significantly compared with placebo. During the resting state of the pelvic floor and during voluntary squeezes, the estimated effect of imipramine versus placebo was 15 cm H2O. These observed pressure increases suggest that imipramine induces a moderate rise in the tone of the anal sphincter complex. To our knowledge, this is the first study to investigate the effect of imipramine on the anal sphincter function, and thus, the mode and site of action have not been established. However, it is likely that the rise in pressure can be attributed to increased stimulation of the striated external anal sphincter via central activation of serotonergic and noradrenergic motor neurons in Onuf’s nucleus in the sacral spinal cord [7, 22].
Physiologically, the AOP reflects the anal canal’s capacity to withstand increased pressure, serving as a metric for the closure function that contributes to continence. Previous studies assessing anal sphincter function using AAR have demonstrated that mean AOP was higher in fecally continent women compared with age-matched incontinent women [12]. Accordingly, the increased resting and squeezing AOP induced by imipramine may potentially improve continence in individuals with FI owing to weak sphincter function. However, the clinical benefit of this potential increase in sphincter tonus remains unknown, particularly when considering the possible adverse effects of imipramine. In our study, 44% of the subjects experienced at least one adverse effect following a single dose of imipramine. The majority of these were anticholinergic and central nervous system effects, resembling the side effects profile reported in longer-term studies [23]. In a broader population, particularly among the elderly and individuals with comorbidities, caution is recommended in the use of TCAs owing to their strong anticholinergic effects, potential for sedation, and risk of orthostatic hypotension [24]. The tolerability is typically better in younger users of imipramine; however, bothersome side effects such as dry mouth, increased heart rate, weight gain, and sexual dysfunction may endure during long-term treatment [23, 25, 26].
In their small, open-label study, Santoro et al. found that low-dose amitriptyline administered over 4 weeks improved FI symptoms and rectal motor activity [6]. The authors attributed these improvements to the anticholinergic properties of amitriptyline, which may prolong gut transit time, thereby enhancing water absorption and normalizing stool consistency. Such prolongation of gut transit has been observed with imipramine in individuals with irritable bowel syndrome and in healthy controls [27, 28]. A recent, randomized, double-blind, placebo-controlled study also confirmed the benefits of low-dose amitriptyline in treating irritable bowel syndrome in the primary care setting [29]. However, amitriptyline, a TCA like imipramine, may influence other pharmacological pathways as well. It may enhance anal sphincter tone by activating serotonergic and noradrenergic receptors in Onuf’s nucleus, as suggested for imipramine. Supporting this, Santoro et al. [6] reported an increase in maximum anal squeeze pressure from a pre-treatment median level of 108 (range 74–278) cmH2O to a median level of 123 cmH2O post-treatment (range not provided). Although not statistically significant, this increase in squeeze pressure matches the increase induced by imipramine in the current study. The lack of statistical significance in the study by Santoro et al. [6] can be attributed to several factors. First, its open-label, uncontrolled design compared pre-treatment and post-treatment values within the same group of individuals with FI, which is less robust than a controlled study. Additionally, the Mann–Whitney U tests, meant for independent samples, were used for dependent samples, reducing the statistical validity and power of the test. Furthermore, the efficacy of amitriptyline was assessed using ambulatory anorectal manometry recordings over variable timeframes, introducing considerable data variability.
In contrast, our study utilized a highly standardized AAR measurement protocol, measuring at specific time points aligned with the peak plasma concentration of imipramine, ensuring high reproducibility and low variability. With this rigorous and standardized AAR technique, we identified a statistically significant change in anal pressure, which aligns with the anticipated effects of imipramine. The within-subject (between-day) SD estimates of the AAR parameters in this study were in line with those reported in previous research (SD for resting AOP: 16.4 versus 14.3 cm H2O in Mitchell et al. [11] and SD for squeezing AOP: 17.5 versus 21 cm H2O in the same study). Additionally, mirroring previous AAR studies, the AAR assessments were feasible, averaging approximately 4 min per session, and well received by the participants. The latter is supported by the absence of adverse events associated with the AAR measurements. Moreover, our analysis confirmed that a placebo effect did not influence these outcomes. Taken together, this suggests that AAR is a reliable technique for assessing the impact of pharmacological treatments on anal sphincter function.
There are limitations to this study that merit consideration. The study population was restricted to healthy women, raising the question of whether imipramine may have a different impact on anal pressure in individuals suffering from FI. Nonetheless, previous clinical studies suggest a good correlation between the increase in urethral pressure caused by certain drugs in healthy women and their efficacy in treating women with stress urinary incontinence. Based on these findings, we believe that this correlation will likely extend to individuals with FI. Furthermore, the effects of imipramine were assessed only once post-administration. Consequently, if the peak plasma concentration of the drug was not achieved at the time of measurement, we might have overlooked its potential influence on AOP. However, earlier UPR studies successfully identified changes in urethral pressure induced by a single dose in healthy women, with the greatest increase coinciding with the estimated time to peak plasma concentration of the study drug [30].
Conclusion
This study demonstrates that a single 50-mg dose of imipramine increases AOP in healthy women, both in the resting state and during voluntary squeezes. The observed increase in AOP suggests that imipramine might potentially improve anal sphincter function. Nevertheless, the clinical impact of these findings remains unknown.
The use of AAR in this study provided reliable and reproducible assessments of anal pressure, supporting its utility in evaluating the impact of pharmacological treatments on anal sphincter function.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Copenhagen University Hospital Bispebjerg and Frederiksberg.
References
Smith TM, Menees SB, Xu X, Saad RJ, Chey WD, Fenner DE. Factors associated with quality of life among women with fecal incontinence. Int Urogynecol J. 2013;24(3):493–9.
Melville JL, Fan MY, Newton K, Fenner D. Fecal incontinence in US women: a population-based study. Am J Obstet Gynecol. 2005;193(6):2071–6.
Assmann SL, Keszthelyi D, Kleijnen J, Anastasiou F, Bradshaw E, Brannigan AE, et al. Guideline for the diagnosis and treatment of Faecal Incontinence—A UEG/ESCP/ESNM/ESPCG collaboration. United European Gastroenterol J. 2022;10(3):251–86.
Menees S, Chey WD. Fecal incontinence. Gastroenterol Clin North Am. 2022;51(1):71–91.
Ehrenpreis EDMD, Chang DBA, Eichenwald EBS. Pharmacotherapy for fecal incontinence: a review. Dis Colon Rectum. 2007;50(5):641–9.
Santoro GA, Eitan BZ, Pryde A, Bartolo DC. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43(12):1676–81.
Thor KB, de Groat WC. Neural control of the female urethral and anal rhabdosphincters and pelvic floor muscles. Am J Physiol Regul Integr Comp Physiol. 2010;299(2):R416–38.
Bharucha AE. Relationship between symptoms and disordered continence mechanisms in women with idiopathic faecal incontinence. Gut. 2005;54(4):546–55.
Klarskov N, Lose G. Urethral pressure reflectometry vs urethral pressure profilometry in women: a comparative study of reproducibility and accuracy. BJU Int. 2007;100(2):351–6.
Klarskov N, Lose G. Urethral pressure reflectometry and pressure profilometry in healthy volunteers and stress urinary incontinent women. Neurourol Urodyn. 2008;27(8):807–12.
Mitchell PJ, Klarskov N, Telford KJ, Hosker GL, Lose G, Kiff ES. Anal acoustic reflectometry: a new reproducible technique providing physiological assessment of anal sphincter function. Dis Colon Rectum. 2011;54(9):1122–8.
Mitchell PJ, Klarskov N, Telford KJ, Hosker GL, Lose G, Kiff ES. Viscoelastic assessment of anal canal function using acoustic reflectometry: a clinically useful technique. Dis Colon Rectum. 2012;55(2):211–7.
Hornung BR, Mitchell PJ, Carlson GL, Klarskov N, Lose G, Kiff ES. Comparative study of anal acoustic reflectometry and anal manometry in the assessment of faecal incontinence. Br J Surg. 2012;99(12):1718–24.
Hornung BR, Telford KJ, Carlson GL, Mitchell PJ, Klarskov N, Kiff ES. Use of anal acoustic reflectometry in the evaluation of men with passive fecal leakage. Dis Colon Rectum. 2017;60(5):521–6.
Kornholt J, Sonne DP, Riis T, Sonne J, Klarskov N. Effect of imipramine on urethral opening pressure: a randomized, double-blind, placebo-controlled crossover study in healthy women. Neurourol Urodyn. 2019;38(4):1076–80.
Malallah MA, Al-Shaiji TF. Pharmacological treatment of pure stress urinary incontinence: a narrative review. Int Urogynecol J. 2015;26(4):477–85.
O’Connor A, Byrne CM, Vasant DH, Sharma A, Liao D, Klarskov N, et al. Current and future perspectives on the utility of provocative tests of anal sphincter function: a state-of-the-art summary. Neurogastroenterol Motil. 2023;35(7):e14496.
Mitchell PJ, Klarskov N, Hosker G, Lose G, Kiff ES. Anal acoustic reflectometry: a new technique for assessing anal sphincter function. Colorectal Dis. 2010;12(7):692–7.
Heywood NA, Nicholson JE, Sharma A, Kiff ES, Klarskov N, Telford KJ. Continuous vs stepwise anal acoustic reflectometry: an improved technique for physiological measurement of anal sphincter function? Neurourol Urodyn. 2020;39(1):447–54.
Senn S. Cross-over trials in clinical research. 1st ed. Chichester/New York: Wiley; 2002. Available from: http://onlinelibrary.wiley.com/doi/10.1002/0470854596
Jones B, Kenward MG. Design and analysis of cross-over trials. Boca Raton: CRC Press/Taylor & Francis; 2014.
Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated: TCAs: pharmacology and interactions. Br J Pharmacol. 2007;151(6):737–48.
Sindrup SH, Bach FW, Madsen C, Gram LF, Jensen TS. Venlafaxine versus imipramine in painful polyneuropathy: a randomized, controlled trial. Neurology. 2003;60(8):1284–9.
2023 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2023 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2023;71(7):2052–81.
Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29(3):259–66.
Mavissakalian M, Perel J, Guo S. Specific side effects of long-term imipramine management of panic disorder. J Clin Psychopharmacol. 2002;22(2):155–61. https://doi.org/10.1097/00004714-200204000-00008
Gorard DA, Libby GW, Farthing MJG. Influence of antidepressants on whole gut and orocaecal transit times in health and irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8(2):159–66.
Gorard DA, Libby GW, Farthing MJG. Effect of a tricyclic antidepressant on small intestinal motility in health and diarrhea-predominant irritable bowel syndrome. Digest Dis Sci. 1995;40(1):86–95.
Ford AC, Wright-Hughes A, Alderson SL, Ow PL, Ridd MJ, Foy R, et al. Amitriptyline at low-dose and titrated for irritable bowel syndrome as second-line treatment in primary care (ATLANTIS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;402(10414):1773–85.
Klarskov N, Cerneus D, Sawyer W, Newgreen D, Van Till O, Lose G. The effect of single oral doses of duloxetine, reboxetine, and midodrine on the urethral pressure in healthy female subjects, using urethral pressure reflectometry. Neurourol Urodyn. 2018;37(1):244–9.
Funding
Open access funding provided by Copenhagen University.
Author information
Authors and Affiliations
Contributions
T. Christoffersen: data analysis, manuscript writing; J. Kornholt: project development, data collection, data analysis, manuscript editing; T. Riis: data collection, data analysis, manuscript editing; D.P. Sonne: data analysis, manuscript editing; N. Klarskov: project development, data collection, data analysis, manuscript editing.
Corresponding author
Ethics declarations
Ethics Statement
Ethical approval was obtained from the Regional Research Ethics Committee of the Capital Region of Denmark (approval no. H‐17007330).
Conflicts of Interest
None.
Additional information
Handling Editor: Rufus Cartwright
Editor in Chief: Maria A. Bortolini
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jonatan Kornholt is an employee of Novo Nordisk, Denmark. However, the ideas and opinions discussed in this publication are those of the author and might not reflect those of his employer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Christoffersen, T., Kornholt, J., Riis, T. et al. Effect of Single-Dose Imipramine on Anal Sphincter Tone in Healthy Women: A Randomized, Placebo-Controlled Study Using Anal Acoustic Reflectometry. Int Urogynecol J (2024). https://doi.org/10.1007/s00192-024-05890-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00192-024-05890-5