Abstract
Introduction and hypothesis
This study is aimed at developing and validating a new integral parameter, the Biomechanical Integrity score (BI-score) of the female pelvic floor for stress urinary incontinence conditions.
Methods
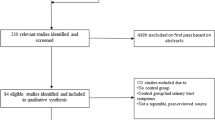
A total of 130 subjects were included in the observational cohort study; 70 subjects had normal pelvic floor conditions, and 60 subjects had stress urinary incontinence (SUI). A Vaginal Tactile Imager (VTI) was used to acquire and automatically calculate 52 biomechanical parameters for eight VTI test procedures (probe insertion, elevation, rotation, Valsalva maneuver, voluntary muscle contractions in two planes, relaxation, and reflex contraction). Statistical methods were applied (t test, correlation) to identify the VTI parameters sensitive to the pelvic SUI conditions.
Results
Twenty-seven parameters were identified as statistically sensitive to SUI development. They were subdivided into five groups to characterize tissue elasticity (group 1), pelvic support (group 2), pelvic muscle contraction (group 3), involuntary muscle relaxation (group 4), and pelvic muscle mobility (group 5). Every parameter was transformed to its standard deviation units using the dataset for normal pelvic conditions, similar to the T-score for bone density. Linear combinations with specified weights led to the composition of five component parameters for groups 1–5 and to the BI-score in standard deviation units. The p value for the BI-score has p = 4.0 × 10–28 for SUI versus normal conditions.
Conclusions
Quantitative transformations of the pelvic tissues, support structures, and functions under diseased conditions may be studied with the SUI BI-score in future research and clinical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary incontinence (UI) is a prevalent condition, affecting more than 1 in 3 women at some point in their lives [1]. More than 20 million women in the USA are affected by UI, and, based on current demographic trends, this number is expected to increase by more than 50% in the coming decades [2]. UI leads to physical, emotional, and social distress, significantly limiting one's lifestyle and ability to engage in work-related activities [3]. The three main types of urinary incontinence are stress, urge, and mixed. Stress urinary incontinence (SUI) occurs when unintended urine leakage appears during coughing, sneezing, or physical exertion [4]. SUI is the most common type overall, but mixed urinary incontinence is the most common type among older women [5]. The prevalence of SUI (defined as any symptoms in the previous year) in adult women is about 46% [6]. Even though UI is widespread, it often goes unnoticed and unreported, with fewer than 40% of affected women seeking medical attention for this issue [7]. It is, therefore, important for health care providers to accurately screen for and diagnose SUI. A thorough medical history is essential for correct diagnosis and assessment. However, it is often difficult to obtain accurate data as patients may be reluctant to talk about their urinary symptoms or be unable to give accurate information about the exact course of the disease, although this information is crucial as it can determine the treatment choice. For this reason, the various questionnaires are helpful in accurately assessing symptoms and disease severity. The Urinary Distress Inventory 6 and Incontinence Impact Questionnaire 7 are most commonly used to assess the symptoms of SUI, whereas the Medical, Epidemiologic, and Social Aspects of Aging (MESA) and the Patient Global Impression of Severity tools are used to assess the severity of the disease [8, 9]. However, further tests may be needed if the answers to the questionnaire are inconclusive. In addition to urinalysis and pelvic examination, urodynamic testing is the cornerstone of diagnosis. In many cases, urodynamic testing is mildly uncomfortable for the patient, but it is an expensive and highly specialized test unsuitable for screening. A common feature of these studies is that they provide little information about etiological factors. The pathology of SUI is complex and influenced by multiple factors. There is substantial evidence suggesting the involvement of bladder neck and urethral incompetence [10, 11]. Additionally, there are indications of compromised urethral support and levator ani muscle function. Therefore, there is a need for standardized measurement approaches to generate more robust and conclusive evidence in this regard [12].
The Vaginal Tactile Imager (VTI) was developed to provide a biomechanical mapping of the pelvic floor with a vaginal probe [13]. A set of new clinical markers/parameters has been proposed for the biomechanical characterization of pelvic floor conditions [14]. This set included 52 parameters automatically calculated as a result of completing eight examination procedures (tests). The Biomechanical Integrity score (BI-score) for pelvic organ prolapse (POP) was proposed [15]. In order to make biomechanical mapping in urogynecology more accessible and valuable, further work is required on developing a shorter list of easily understandable and practical biomechanical parameters for SUI characterization.
This article is aimed at reporting the development and validation of a new integral parameter, the BI-score, for characterizing the female pelvic floor under SUI.
Materials and Methods
Definitions
Tactile imaging is a medical imaging modality that translates the sense of touch into a digital image [13]. The tactile image is a function of P(x,y,z), where P is the pressure on the soft-tissue surface under applied deformation, and x, y, and z are the coordinates where P was measured. The tactile image is a pressure map on which the direction of tissue deformation must be specified.
Functional tactile imaging translates muscle activity into the dynamic pressure pattern P(x,y,t) for an area of interest, where t is time and x and y are coordinates where the pressure P was measured. It may include a muscle voluntary contraction, an involuntary reflex contraction, involuntary relaxation, and specific maneuvers.
A tactile imaging probe has a pressure sensor array mounted on its face that acts in similar manner to human fingers during a clinical examination, deforming the soft tissue and detecting the resulting changes in the pressure pattern on the surface. The sensor head is moved against or over the surface of the tissue to be studied, and the pressure response is measured at multiple locations along the tissue. The results are used to generate images that show pressure distribution over the area of the tissue under study. The tactile image P(x,y,z) reveals tissue or organ anatomy and elasticity distribution [16, 17].
Vaginal Tactile Imager
The VTI, model 2S, was used for biomechanical mapping of the pelvic floor. As shown in Fig. 1, the VTI probe is equipped with 96 pressure (tactile) sensors spaced consecutively on both sides of the probe, an orientation sensor, and temperature controllers to provide the probe temperature close to a human body before the examination. During the clinical procedure, the probe is used to acquire pressure responses from two opposite vaginal walls (anterior–posterior and left–right) along the vagina. The VTI data are sampled from the probe sensors and presented on the VTI display in real time. The resulting pressure maps (tactile images) of the vagina integrate all the acquired pressure and positioning data for each pressure-sensing element during vaginal wall deformation and pelvic muscle contraction. Lubricating gel is used for patient comfort. It also provides reproducible boundary/contact conditions with deformed tissues.
The VTI examination procedure consists of eight tests. The spatial gradients ∂P(x, y)/∂y (changes of pressure along the tissue deformation per 1 mm) for the anterior and posterior compartments are calculated within the acquired tactile images in tests 1 and 2; the y-coordinate is directed orthogonally from the vaginal channel coming through the anterior–posterior compartments, and the x-coordinate is located on the vaginal channel.
The VTI probe is calibrated with reference pressure sensors (Honeywell Inc., Charlotte, NC, USA) immediately before every subject examination. The VTI absolute measurement accuracy is as follows: ± 0.2 kPa within the 10 kPa range, ± 0.5 kPa at 25 kPa, and ± 1.0 kPa at 60 kPa. The VTI relative pressure measurement accuracy ranges from ± 0.05 kPa to ± 0.1 kPa. The intra- and inter-observer reproducibility of vaginal tactile imaging was reported earlier [18]. Intra-observer intraclass correlation coefficients (ICCs) were found in the range from 0.80 (test 8: cough) to 0.92 (test 3: rotation) with an average value of 0.87. Inter-observer ICCs ranged from 0.73 (test 2 elevation pressure and test 8 cough) to 0.92 (test 3 rotation), with an average value of 0.82. Intra-observer limits of agreement ranged from ± 11.3% (test 1) to ± 19.0%% (test 8) with an average value of ± 15.1%. Inter-observer limits of agreement ranged from ± 12.0% (test 5 voluntary contraction) to ± 26.7% (test 2 elevation) with an average value of ± 18.4%. These numbers lead to the projection of reproductivity for the BI-score and its components in the range from ± 0.1 to ± 0.2 standard deviation. Improved inter-observer reproducibility is possible through additional operator training and consistency in the VTI examination technique. The VTI pressure measurement resolution is 10 Pa. The VTI absolute measurement accuracy for probe orientation is ± 0.5 °C and ± 0.1 °C for measuring the temperature inside the probe on the surface of the pressure sensors. The tactile images and muscle contraction patterns are visualized with a resolution of 1.0 mm [13].
Biomechanical Parameters
The complete list of 52 VTI biomechanical parameters, their interpretation, and anatomical assignments of the targeting/contributing pelvic structures into the specified parameters are presented in a previous publication [14].
The parameters listed in Table 1 have different units (see the fourth column in Table 1). The next step was to bring all the selected parameters to uniform units to allow their arithmetic combination. Among various possible options, the preference for the units of standard deviation was provided (see the explanation pertaining to such selection in the Discussion section). All VTI data were transformed according to Eq. 1 below.
Here, \({Po}_{n}^{i}\) is an original value of the n-parameter for the i-subject; \({Pa}_{n}\) is an arithmetic average of the n-parameter for subjects aged 18–39 years in the group with a normal pelvis (70 subjects); \({SD}_{n}\) is a standard deviation for the n-parameter for 70 subjects in the group with a normal pelvis, and \({Psd}_{n}^{i}\) is the transformed value of the n-parameter for the i-subject in units of standard deviation. Now, we can combine the parameters expressed in units of standard deviation using a linear operation of addition. First, the 27 selected parameters were subdivided into five groups. We may call them by the five components to characterize: tissue elasticity (component 1), pelvic support (component 2), pelvic muscle contraction (component 3), muscle relaxation (component 4), and muscle mobility (component 5; Fig. 2). Component 1 comprises seven parameters with weights of 0.143, component 2 comprises eight parameters with weights of 0.125, component 3 consists of eight parameters with weights of 0.125, component 4 comprises two parameters with a weight of 0.5, and component 5 consists of two parameters with a weight of 0.5. It is crucial that VTI parameters 28 and 52 were used with negative signs because they have increased values at SUI versus normal conditions (Table 1). Finally, these five components create the BI-score with equal weights of 0.2, as shown in Fig. 2.
Study Population
The analyzed dataset in this study includes subjects with normal pelvic floor and SUI from two VTI clinical studies with identical VTI examination procedures. The subjects were examined with the VTI in the scope observational cohort studies completed from May 2022 to June 2023 (study 1) and June 2020 to September 2021 (study 2). Similar clinical protocols were approved by the Institutional Review Board (study 1: Western IRB and local IRB as required; study 2: Scientific and Research Ethics Committee of Hungary: 2876–13/2022/EÜIG), and written informed consent was obtained from all the subjects enrolled in the studies. Table 2 presents the mean and standard deviation for the subject age, parity, weight, and height separately for the normal and POP groups. The VTI examination data for the eight tests were obtained and recorded at the time of the scheduled urogynecology visits [14]. All the analyzed subjects needed not to have had any prior pelvic surgery.
The total study workflow comprised the following steps:
-
1.
Recruiting women who did not previously have pelvic surgery and had normal pelvic floor conditions (no SUI) or had SUI measured by the Medical, Epidemiologic, and Social Aspects of Aging (MESA) questionnaire [8]
-
2.
Acquiring clinical diagnostic information related to the cases included in the study by standard clinical means
-
3.
Performing a VTI examination in a lithotomy position
-
4.
Analyzing VTI data
Study exclusion criteria were active infection or ulceration within the vagina; presence of a vaginal septum; active cancer of the colon, rectum wall, cervix, vaginal, uterus, or bladder; ongoing radiation therapy for pelvic cancer; impacted stool; significant pre-existing pelvic pain, including levator ani syndrome, severe vaginismus, or vulvodynia; severe hemorrhoids; significant circulatory or cardiac conditions that could cause excessive risk from the examination, as determined by the attending physician; and current pregnancy. In study 2, which targets normal pelvic conditions, one inclusion criterion was modified to women aged 18–39 years, and two exclusion criteria were added as follows: the woman is a regular patient visiting the urogynecology clinic (two or more times during the last year); and cognitive impairment.
Statistical Methods
A total of 52 biomechanical parameters were calculated automatically by the VTI software version 2023.66.1.0 per each of the 130 analyzed VTI examination data. The two-sample t test (p < 0.01) was employed to test the null hypothesis that the data in normal and SUI groups have equal means and equal variances. The Bonferroni method was used to adjust for multiple comparisons, changing the commonly accepted statistical p value from 0.05 to 0.01. The alternative hypothesis is that the data in these groups come from populations with unequal means. The p values for testing the hypothesis were calculated. Pearson's linear correlation coefficients (r) were calculated among 52 VTI parameters, each parameter against all other 51 parameters.
To visually evaluate the analyzed data distributions, we used notched boxplots showing a confidence interval for the median value (central vertical line), and 25% and 75% quartiles [19]. The spacing between the different parts of the box helps to compare variance. The boxplot also determines skewness (asymmetry) and outliers (cross). The statistical functions of MATLAB, version R2022b (MathWorks, MA, USA), were used for the data analysis.
Results
In this study of 130 subjects, 70 subjects had normal pelvic floor conditions, and 60 subjects had SUI according to MESA score. The mean subject age and parity in the normal and SUI groups are significantly different: 27.7 versus 54.6 years old and 0.5 versus 1.9 respectively. The mean subject weight and height are the same in both groups (Table 2). The last column in Table 1 brings p values for the two-sample t tests (normal versus SUI). The p values for the VTI parameters are found in the range of 2.1 × 10 − 18 to 1.1 × 10 − 2, with most p values being < 1.0 × 10 − 5. The p value for the BI-score has p = 4.0 × 10 − 28 for the two analyzed groups.
We first aimed to select VTI parameters with significant changes at SUI versus the normal pelvic conditions during the statistical analysis. Two specific quantitative criteria were imposed on such selection: a t test p < 0.01 for the dataset of 60 SUI cases against the dataset for 70 normal cases; and a correlation coefficient r < 0.85 with all other parameters. The first criterion passed 43 parameters; both the first and second 27 parameters. Figure 3 presents the boxplots, and Table 1 shows the numerical data for these selected 27 VTI parameters responsive to SUI and not highly correlated with each other. For consistency, the numbering of the VTI parameters in this article is kept exactly as in earlier publications [14].
All the BI-score data for 130 subjects analyzed here can be visualized on one graph as a function of the subject's age (see left panel in Fig. 4). The dashed lines show ± 1.0 standard deviation for normal pelvic conditions from the reference (zero) line. The right panel in Fig. 4 shows the same BI-score data in two boxplots for normal and SUI pelvic conditions. One may observe a significant separation between these two groups; the t test gives p = 4.0 × 10–28 for these two groups.
Discussion
The BI-score is the composite score that consists of five components, as shown in Fig. 2. These five components bring different aspects of the biomechanical characterization of the pelvic floor. Owing to excluding the highly correlated original VTI parameters with r ≥ 0.85, the mutual correlation coefficients have an average value of r = 0.25, which is considered low. It is important to note that the tissue elasticity component integrates the tissue/structure elasticity for the 0- to 8-mm layer behind the vaginal walls from the depth comparative with the vaginal wall deformations in tests 1 and 3 (see Fig 2 and parameter interpretation in Egorov et al. [14]). The pelvic support component integrates the structure support from a depth of 5–45 mm, which is about the same as the vaginal wall deformations in test 2 (see Fig. 2 and parameter interpretation in Faulkner [20]).
Table 1 shows VTI parameter changes in SUI relative to the normal pelvic conditions. In SUI the elasticity parameters (−50.7% to −64.9%), the pelvic support parameters (−41.3% to −78.7%), and the muscle contraction parameters (−60.2% to −71.3%) are lower than in normal pelvic conditions. The muscle relaxation speed parameters, which have a negative sign because muscle force involuntarily goes down, are higher (131.5% to 152.8%) in SUI—relaxation develops faster.
The mean age and parity of the subjects in the normal and SUI groups are significantly different, which is the intended difference in the groups analyzed, because for the reference (zero line in the BI-score), we need a young population without SUI, which develops with age. As mentioned above, most of the p values of the VTI parameters (normal versus SUI) are < 1.0 × 10−5, and the p value for the BI-score is p = 4.0 × 10–28 for the two groups analyzed. It indicates that the data in these groups come from populations with unequal means and strong sensitivity to SUI. These results can be considered statistically significant validation for the BI-score sensitivity to SUI. Further, the SUI BI-score range can be subdivided into three zones—normal, transitional, and diseased—similar to what was suggested for the POP BI-score [11]. As with bone density measurement, monitoring patient progress with or without treatment is essential. Moreover, for this reason, it would be great to define the minimal clinically important difference in BI-score. The future research directions may also address:
-
1.
BI-score use for monitoring a pelvic floor treatment outcome
-
2.
Obtaining a periodic BI-score before a woman has symptoms
-
3.
Recommendation for specific treatment based on the five components (e.g., treatment for elasticity is needed but not for relaxation or muscle mobility
-
4.
Predictive capabilities of the BI-score for symptoms (e.g., a woman is less or more likely to develop some form of pelvic floor dysfunction)
These crucial questions are beyond this article.
The strength of this study is that the suggested BI-score covers biomechanical aspects of the pelvic floor, including tissue elasticity, pelvic support, muscle contraction, involuntary relaxation, and mobility. All these aspects usually deteriorate as the pelvic disease develops. This quantitative characterization can be used in diagnosing and monitoring pelvic conditions and selecting and justifying a treatment.
The weakness of this study is the absence of statistically significant results for possible variations in ethnicity and race, which must be the subject of future research. Also, thousands of new VTI examinations for normal pelvic floor conditions may adjust the mean and standard deviation values used in Eq. 1 for BI-score calculations. In addition, this was a cross-sectional study rather than a prospective longitudinal study, where disease processes can be assessed over time.
Conclusion
Based on the analysis conveyed, five components, (tissue elasticity, pelvic support, pelvic muscle contraction, muscle relaxation, and muscle mobility) are vital for biomechanically characterizing the pelvic floor and particularly useful for describing biomechanical changes in women with and without SUI. All these components contribute to the integral parameter of the BI-score. Objectively measurable transformations of the pelvic tissues, support structures, and functions under different diseased conditions may be studied with the BI-score in future research and practical applications.
References
Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses’ Health Study. Am J Obstet Gynecol. 2003;189:428–34.
Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstet Gynecol. 2009;114:1278–83.
Wu JM. Stress incontinence in women. N Engl J Med. 2021;384:2428–36.
Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21:5–26.
Minassian VA, Bazi T, Stewart WF. Clinical epidemiological insights into urinary incontinence. Int Urogynecol J. 2017;28:687–96.
Abufaraj M, Xu T, Cao C, et al. Prevalence and trends in urinary incontinence among women in the United States, 2005–2018. Am J Obstet Gynecol. 2021;225:166.e1–12.
Waetjen L, Xing G, Johnson W, Melnikow J, Gold E. Factors associated with reasons incontinent midlife women report for not seeking urinary incontinence treatment over 9 years across the menopausal transition. Menopause. 2018;25:29–37.
Diokno AC, Brock BM, Brown MB, Herzog AR. Prevalence of urinary incontinence and other urological symptoms in the noninstitutionalized elderly. J Urol. 1986;136:1022–5.
Yalcin I, Bump RC. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynecol. 2003;189:98–101.
DeLancey JO. Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol. 1994;170:1713–23.
Petros PE, Ulmsten UI. An integral theory of female urinary incontinence. Experimental and clinical considerations. Acta Obstet Gynecol Scand Suppl. 1990;153:7–31.
Falah-Hassani K, Reeves J, Shiri R, Hickling D, McLean L. The pathophysiology of stress urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J. 2021;32:501–52.
Egorov V, van Raalte H, Lucente V, Sarvazyan A. Biomechanical characterization of the pelvic floor using tactile imaging. In: Hoyte L, Damaser M, editors. Biomechanics of the female pelvic floor. Cambridge MA: Academic; 2016. p. 317–48.
Egorov V, Shobeiri SA, Takacs P, Hoyte L, Lucente V, van Raalte H. Biomechanical mapping of the female pelvic floor: prolapse versus normal conditions. Open J Obstet Gynecol. 2018;8:900–24.
Egorov V, van Raalte H, Takacs P, Shobeiri SA, Lucente V, Hoyte L. Biomechanical integrity score of the female pelvic floor. Int Urogynecol J. 2022;33:1617–31.
Egorov V, Sarvazyan AP. Mechanical imaging of the breast. IEEE Trans Med Imaging. 2008;27:1275–87.
Egorov V, Ayrapetyan S, Sarvazyan AP. Prostate mechanical imaging: 3-D image composition and feature calculations. IEEE Trans Med Imaging. 2006;25:1329–40.
Van Raalte H, Lucente V, Ephrain S, et al. Intra- and inter-observer reproducibility of vaginal tactile imaging. Urogynecology. 2016;22:S130–1.
Mcgill R, Tukey JW, Larsen WA. Variations of box plots. Am Stat. 1978;32:12–6.
Faulkner KG. The tale of the T-score: review and perspective. Osteoporos Int. 2005;16:347–52.
Acknowledgements
The US Army Medical Research Acquisition Activity, 839 Chandler Street, Fort Detrick, MD 21702-5014, USA, is the awarding and administering acquisition office. Research reported in this publication was supported by the National Institutes of Health under Awards Numbers R44HD097805, R43HD109075, and by the Department of Defense (DoD), through the Broad Agency Announcement (BAA), for Extramural Medical Research, under Award No. W81XWH1920018. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense and the National Institutes of Health.
Funding
Open access funding provided by University of Debrecen.
Author information
Authors and Affiliations
Contributions
P.T.: clinical protocol development, data collection and analysis, manuscript writing and review; D.R.: data collection and analysis, manuscript editing and revision; E.K.: data collection and analysis, manuscript editing and revision; H.v.R.: clinical data collection and analysis, manuscript editing and review; V.L.: clinical data collection and analysis, manuscript editing and revision; V.E.: device design and assembling, imaging software development, data analysis, manuscript editing; Z.T.K.: clinical protocol development, data collection and analysis, manuscript editing and review; B.K.: clinical protocol development, data collection and analysis, manuscript writing.
Corresponding author
Ethics declarations
Conflicts of Interest
V.E. is a CEO and a minor shareholder in Advanced Tactile Imaging, Inc. H.v.R. is a minor shareholder of Advanced Tactile Imaging, Inc. P.T., D.R., E.K., V.L., Z.T.K., and B.K. report no conflicts of interest.
Additional information
Handling Editor: Jaromir Masata
Editor in Chief: Maria A. Bortolini
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takacs, P., Rátonyi, D., Koroknai, E. et al. Biomechanical Integrity Score of the Female Pelvic Floor for Stress Urinary Incontinence. Int Urogynecol J 35, 1245–1253 (2024). https://doi.org/10.1007/s00192-024-05797-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-024-05797-1