Abstract
Introduction and hypothesis
Polypropylene (PP) mesh for the treatment of pelvic organ prolapse (POP) has raised substantial concerns over long-term complications, leading to its ban in multiple countries. In response, emerging materials are being explored as alternatives for prolapse surgery. Preclinical animal models have historically played a pivotal role in validating medical devices, prior to clinical trials. Successful translation of these materials necessitates the identification of suitable animal models that replicate the female human pelvis and its biomechanical properties. Preclinical in vivo testing assesses the safety of surgical mesh and treatment efficacy in preventing POP recurrence.
Methods
The research critically reviews animal models used for preclinical pelvic mesh testing over the last decade and proposes a promising model for future preclinical studies.
Results
Rats were the most common mammal used for toxicity and biocompatibility investigations through abdominal implantation. Although non-human primates serve as a gold standard for efficacy testing, ethical considerations limit their use owing to their close biological and cognitive resemblance to humans. Consequently, sheep were the most preferred large animal model owing to their reproductive system similarities and propensity for spontaneous POP following parity.
Conclusion
The study contributes valuable insights into the selection of appropriate animal models for preclinical pelvic mesh testing, offering guidance that is crucial for enhancing the safety and efficacy of novel surgical interventions in the treatment of POP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of animal models in clinical research traces back to ancient Greece and dates back to the sixth century BCE. Initially employed to enhance our understanding of clinical anatomy and serve as comparative models for studying mammalian anatomy, animal models have played a pivotal role in the evolution of scientific research. Over the centuries, the role of animal models has extended beyond anatomical studies to encompass a wide range of applications, including preclinical trials for testing medical drugs, surgical devices and even products in industries such as cosmetics. This has led to the animal studies industry becoming a billion-dollar market, with health care companies now relying on animal models to evaluate product safety, toxicity and efficacy prior to human translation.
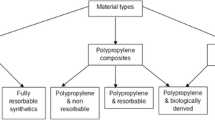
The polypropylene (PP) mesh (Fig. 1) is a surgical adjunct used to treat pelvic organ prolapse (POP). The transvaginal PP mesh was initially approved in 1996, having been modelled from the mesh previously used in hernia surgery. However, concerns surrounding long-term complications, such as chronic pain and mesh exposure, have prompted the ban of transvaginal PP meshes in several countries, including the UK, USA and Canada. To introduce a novel surgical implant onto the market, extensive testing is imperative to ensure product safety prior to human use, involving meticulously designed preclinical trials. In this context, it becomes paramount to design appropriate animal trials and select the most suitable animal model that closely mimics the biomechanical properties and histology of the female human pelvis.
The primary objective of this research was to critically review the animal models used in preclinical pelvic mesh testing to date, aiming to propose the most suitable animal model for the design of future preclinical trials investigating novel surgical implants for prolapse surgery. Our review will highlight preclinical animal trials investigating the application of PP mesh in POP treatment over the last decade and assess the suitability of each animal model. In these in vivo trials, we aim to assess not only the safety of the mesh surgical adjunct in avoiding long-term complications but also its efficacy in preventing recurrence of POP.
Materials and Methods
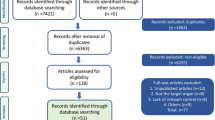
For this comprehensive review, we conducted a systematic search of three major databases: PubMed/Medline, Embase and Cochrane Library (Wiley). Our search was aimed at identifying relevant studies on animal models in POP research and the use of synthetic mesh. We employed the following key words: animal model; pelvic organ prolapse; pelvic mesh; synthetic mesh; and a range of animal species often used in preclinical trials. We restricted the search to publication over the last 10 years.
Results
A summary of all preclinical trials utilising animal models to investigate the safety and efficacy of synthetic pelvic mesh has been highlighted in Table 1. Overall, we identified 36 animal trials dedicated to investigating synthetic mesh for POP in the past decade. In subsequent sections, we comprehensively assess the suitability of each animal model used.
Mouse
Mice are small, readily available and cheap, and are hence commonly used in preclinical trials. However, their suitability depends on the purpose of the trial. Owing to their small size, housing is easy. Mice may be used initially to investigate the toxicity of the implant material prior to testing efficacy in larger animal models.
The mouse reproductive tract differs from the human reproductive tract in a number of ways. A significant structural difference is that midline fusion of Müllerian ducts leads to a unicornuate uterus in humans and a bicornuate uterus in mice [37]. Researchers must be mindful of these anatomical differences when designing experiments or interpreting results, especially if the study involves aspects of the reproductive tract that are influenced by uterine structure. This structural difference makes mice unsuitable for the mechanical investigation of a novel surgical adjunct for POP.
Of all 36 trials, only two of the trials of the last 10 years used mice for preclinical tests. Both of the preclinical trials that took place in mice investigated the use of endometrial stem cells to enhance tissue regeneration and recovery [17, 21]. The findings of both concluded that endometrial mesenchymal stem cells improved clinical outcomes and that gelatin improved retention of these stem cells. Mice were deemed most suitable for these studies as the breed of mouse used (NOD SCID gamma mice) lacks an adaptive immune system and allows focus on the innate foreign body response. In addition, this breed has a longer retention period of stem cells.
Non-Human Primate
Non-human primates (NHPs) are the gold standard animal model for preclinical trials investigating a surgical adjunct for POP repair surgery. NHPs, especially rhesus macaques, share several anatomical and physiological similarities with humans, making them an appropriate model for studying pelvic anatomy. For example, their bipedal walking, squatting behaviour on defecation and vaginal delivery of live infants are characteristics that closely resemble those of human activities. Thus, the NHP is an appropriate model for investigating the efficacy of a pelvic implant.
The rhesus macaque is an NHP species considered most similar to the female human with regard to pelvic anatomy and mechanical properties. Rhesus macaques experience pelvic remodelling during pregnancy, primarily because of the larger head size of their infants—similar to humans [38]. This remodelling process, particularly in multiparous individuals, increases their susceptibility to spontaneous POP. This characteristic makes them even more relevant for POP research.
Two animal studies of the last 10 years used NHPs, experimenting with commercial PP mesh (Gynemesh™) and MatriStem as treatments for POP [18, 30]. Both the aforementioned studies used the rhesus macaque species for their investigations. Both these studies were carried out by the same research team and the research has not yet been brought forward into clinical trials. No results have yet been found investigating the use of MatriStem to treat POP.
Despite their suitability as animal models for preclinical trials, NHPs are less commonly used in research owing to several challenges. These include high cost, difficulty in obtaining and housing, and ethical concerns associated with use in biomedical research. In summary, although NHPs provide a valuable and relevant model for investigating the efficacy of pelvic implants for POP repair, their use is limited by practical and ethical considerations. Nonetheless, their anatomical and physiological similarities to humans make them a valuable resource for understanding and developing treatments for POP.
Ovine
Ovine, or sheep, are large animals abundant and readily available from the farming industry, making them relatively affordable as a model for large animal studies. Their availability and cost-effectiveness contribute to their frequent use in research. Sheep are unique among quadrupedal mammals in that they can develop spontaneous POP. This phenomenon is believed to be related to their delivery of large live infants [39, 40]. Understanding and investigating spontaneous POP using sheep can be crucial to providing insights into treatment and cure in humans.
Ovine pelvic anatomy is considered most similar to that of humans compared with other quadrupedal mammals [22]. This similarity extends to histology, vaginal size and collagen composition [41]. This makes sheep a valuable model for studying POP in relation to human anatomy. Figure 2 depicts the sheep reproductive system, showing the uterine horns and body, the cervix as pointed out by the instrument, leading inferiorly to the vagina.
Sheep were the most popular large animal model, with 7 of the 36 animal trials noted in Table 1 being performed in sheep. These trials likely chose sheep because of their anatomical similarities to humans and their propensity to develop POP. However, this does not mean that working with sheep as an animal model does not carry its own downsides and challenges.
One challenge associated with using sheep in preclinical trials is the difficulty in anaesthesia and positioning. Sheep have a non-straight back, which goes on to complicate surgical procedures when placing these animal models in a dorsal position; to overcome this the procedure would have to be performed as fast as possible whilst maintaining accuracy [42]. In addition, continuous gastric secretions during the procedure cause problems, with the possibility of post-operative complications. It is important to consult a veterinary specialist prior to performing any procedure.
One particularly promising aspect of using sheep as a model is that some research groups have successfully transitioned from preclinical trials in sheep to clinical trials in humans. A research group in China carried out initial investigations in the ovine model and continued to human clinical trials using their titanium-coated PP mesh, TiLOOP [43]. Note that this was the only animal study of a novel implant for POP identified to have been carried on into human clinical trials. This suggests that the sheep model has translational potential for preclinical studies.
In summary, ovine models offer anatomical and physiological similarities to humans, making them valuable in preclinical trials for POP treatments. Although there are challenges related to anaesthesia and positioning, the translational potential demonstrated in some studies underscores the significance of this animal model in advancing POP research.
Porcine
Porcine or pig models have been among the least commonly used animal models for preclinical trials investigating surgical adjuncts for POP repair surgery. The porcine model was used for only 2 of the 36 studies identified in Table 1, which is the same frequency as the use of the mouse model and NHPs [2, 7]. These studies were both carried out by the same research group. One advantage highlighted by the research group was the larger size of the pig vagina. This larger size allowed them to utilise several implants per animal for their studies. This can be advantageous for particular experimental set-ups, especially when the quantity is significant.
A notable challenge associated with the use of pigs is their growth pattern. Pigs continue to have growth spurts until sexual maturity is reached, which can create additional costs and logistical challenges related to handling and housing. This continuous growth can affect the stability of mechanical properties, potentially impacting the reliability of the study results. Owing to the challenges related to growth and associated costs, pigs are generally considered less desirable for research purposes. The abundance of pigs in the farming industry does not make them the preferred choice for preclinical studies.
In summary, although porcine models have been used in a limited number of preclinical studies for investigating POP treatments. Their growth pattern and associated challenges make them less desirable for this purpose than other animal models, such as sheep. Researchers need to consider the potential impact of growth-related variability on study outcomes when using porcine models in such surgical research.
Rabbit
The rabbit serves as a valuable animal model for conducting initial toxicology and biocompatibility studies related to surgical implants for POP. However, its essential to note that rabbits are not known to suffer from POP and therefore the rabbit model is not an appropriate model for testing treatment efficacy of a novel implant [44]. Despite this, rabbits have some desirable qualities in that they are relatively larger in size than other rodents, such as rats [5]. The larger overall size of rabbits provides researchers with a more feasible platform for investigating POP grafts.
The rabbit has an internal abdominal vagina and an external vagina. The external vagina is better accessible for surgical procedures requiring pelvic access. Rabbit was used in only 8 of the studies outlined in Table 1. Because rabbits are larger than rats, vaginal implantation was observed to be the main benefit [5]. However, it is noted that of the 8 studies performed in rabbits, only 3 of these studies opted to use the vaginal site to investigate the mesh graft [5, 6, 16]. Future research may explore methods of using rabbit models in ways that benefit researchers and allow reliability of results, by way of testing biocompatibility and toxicology at the site of vaginal tissue.
Rats
Rats emerged as the most frequently used animal model, featured in 17 out of the 36 trials. This is due to a number of advantageous and desirable qualities of the rat model. Rats are small and do not grow significantly, are thus cost-effective and easy to feed, handle and house, and do not require much space. Rats are also readily available, thus overall providing a practical option.
The small size of the rat model does make it an economical choice, as smaller sections of implant material can be investigated per animal model. However, this prevents investigation of efficacy. The small size of rats also imposes limitations on the level of tension that can be applied when investigating pelvic mesh, thereby restricting the scope of investigations [10]. Although the rat model cannot be used to test treatment efficacy, it is readily available for toxicology and biocompatibility.
Rat connective tissue composition is similar to that of humans [44]. Other research has found the rat model more suitable for pelvic floor studies than mouse or rabbit, because there are more similarities to the human pelvis [45]. A common site on the rat model for device implantation is in the subcutaneous tissue of the abdominal wall [46]. This site provides an easier surgical procedure and a large surface area for explant analysis.
In conclusion, rats serve as valuable subjects in the early stages of preclinical trials for the investigation of new materials. Desirable qualities include affordability, accessibility, and tissue composition similarities to humans. Rats provide a suitable benchmark for initial studies of material toxicity and biocompatibility, prior to continuing preclinical tests of treatment efficacy in larger animals.
Discussion
The human pelvis has unique features that support upright bipedal transport, which thus has a significant impact on pelvic environment. These features play a crucial role in designing preclinical trials to assess the efficacy of surgical implants for treating POP. Replicating the biomechanical environment of the human pelvis closely in in vivo investigations is essential for ensuring successful human translation. NHPs are considered the gold standard animal for these preclinical trials, but their use is limited owing to high costs and ethical concerns. Preclinical guidelines categorise NHPs as “acutely scarce resources” [30]. Therefore, academic and industry researchers must conduct investigations using alternative suitable models.
The “3Rs” principle, developed over 50 years ago, provides a framework for responsible animal research. The principle advocates the reduction, replacement and refinement of animals included in preclinical in vivo investigations. Reduction translates to using the minimum number of animals for consistent results. Refinement means not causing the animals any unnecessary harm. Replacement is to replace animals with other modes of investigation, such as numerical and computer modelling [47]. This framework is aimed at minimising animals used whilst ensuring consistent and reliable results. An alternative, in order to reduce and refine the use of animal models, would be to encourage further use of ex vivo studies prior to in vivo investigations.
The choice of the animal model for preclinical investigations should align with whether the focus is on biocompatibility/toxicity studies versus treatment efficacy. Rats are a common animal model for biocompatibility and toxicity studies, across all specialities. This is because they are small and easy to house and maintain. Only the material is being investigated; therefore, a small amount can be implanted subcutaneously. Owing to the frequent usage of rats in biocompatibility studies, established protocols exist providing consistency for biocompatibility and toxicity studies.
The anatomical structures and organ sizes are important to prepare for when planning animal trials. The adult human uterus measures approximately 8cm in height, 5cm in width and 3cm in thickness, with variations amongst individuals, parity, and stage of the menstrual cycle. Smaller animals, such as mice, have much smaller pelvic dimensions, therefore limiting the amount of material available for use in the model. Therefore, efficacy investigations require large animal models.
With regard to efficacy, sheep models provide the most suitable large animal model for efficacy investigations. A degree of error needs to be considered owing to bipedal versus quadrupedal locomotion. In addition, most animals, including sheep, are structured to support tails and tail function with muscles pointed dorsally, converse to the human pelvis [48]. The sheep have been recorded to suffer from spontaneous POP, as mentioned above, and so provide a useful, replicable environment for POP investigation.
Conclusions
In this paper, we performed a thorough investigation of the animal models used to investigate a surgical adjunct for the treatment of POP. Six animal models were identified from preclinical trials spanning over the last decade, including mice, non-human primates, pigs, sheep, rabbits, and rats. Each animal model was discussed in detail highlighting the benefits and downsides of use. We concluded that rats were the most frequently used species, owing to their small size and the fact that they are readily available. The gold-standard animal model is the non-human primate; however, this is rarely used in reality owing to ethical concerns and limited availability. Sheep were the most common large animal model, as they provide a suitable alternative and are known to develop spontaneous POP. Preclinical trials are critical to evaluating the safety and efficacy of a device prior to human translation and careful selection of the animal model and design of the trial is significant to the translatability of outcomes.
Data availability statement
Data sharing is not applicable. No new data were created or analysed in this study. Data sharing is not applicable to this article.
References
Liang R, Fisk A, King G, Meyn L, Xiao X, Moalli P. Characterization of vaginal immune response to a polypropylene mesh: diabetic vs. normoglycemic conditions. Acta Biomater. 2022;143:310–9. https://doi.org/10.1016/j.actbio.2022.03.007.
Kisby CK, Shadrin IY, Rolland TJ, et al. Exosome-induced vaginal tissue regeneration in a porcine mesh exposure model. Female Pelvic Med Reconstr Surg. 2021;27:609–15.
Li H, Shu H, Qiao G, Dai Z. Visualization of implanted mesh in the pelvic reconstructive surgery using an X-ray-detectable thread. Arch Gynecol Obstet. 2021;304:965–73. https://doi.org/10.1007/s00404-021-06180-x.
Morch A, Doucède G, Lecomte-Grosbras P, Brieu M, Rubod C, Cosson M. Pelvic organ prolapse meshes: can they preserve the physiological behavior? J Mech Behav Biomed Mater. 2021;120:104569. https://doi.org/10.1016/j.jmbbm.2021.104569.
Peró M, Casani L, Castells-Sala C, Pérez ML, et al. Rabbit as an animal model for the study of biological grafts in pelvic floor dysfunctions. Sci Rep. 2021;11:10545. https://doi.org/10.1038/s41598-021-89698-z.
Knight KM, King GE, Palcsey SL, Artsen AM, Abramowitch SD, Moalli PA. A soft elastomer alternative to polypropylene for pelvic organ prolapse repair: a preliminary study. Int Urogynecol J. 2022;33:327–35. https://doi.org/10.1007/s00192-021-04792-0.
Kisby CK, Shadrin IY, Peng LT, et al. Impact of repeat dosing and mesh exposure chronicity on exosome-induced vaginal tissue regeneration in a porcine mesh exposure model. Female Pelvic Med Reconstr Surg. 2021;27:195–201.
Bickhaus JA, Fraser MO, Weidner AC, et al. Polycarbonate urethane mesh: a new material for pelvic reconstruction. Female Pelvic Med Reconstr Surg. 2021;27:e469–75.
Bickhaus JA, Fraser MO, Weidner AC, et al. Evaluation of host immune cellular and extracellular matrix responses to prolapse mesh with and without tension in a rat model. Female Pelvic Med Reconstr Surg. 2021;27:e385–91.
Eisenakh IA, Bondarev OI, Mozes VG, Lapii GA, Lushnikova EL. Features of in vitro degradation and physical properties of a biopolymer and in vivo tissue reactions in comparison with polypropylene. Bull Exp Biol Med. 2020;170:88–92. https://doi.org/10.1007/s10517-020-05010-5.
Deng M, Ding J, Ai F, Mao M, Zhu L. Impact of human umbilical cord-derived stem cells (HUMSCs) on host responses to a synthetic polypropylene mesh for pelvic floor reconstruction in a rat model. Cell Tissue Res. 2020;382:519–27. https://doi.org/10.1007/s00441-020-03234-5.
Mori da Cunha MGMC, Arts B, Hympanova L, et al. Functional supramolecular bioactivated electrospun mesh improves tissue ingrowth in experimental abdominal wall reconstruction in rats. Acta Biomater. 2020;106:82–91. https://doi.org/10.1016/j.actbio.2020.01.041.
Emmerson S, Mukherjee S, Melendez-Munoz J, et al. Composite mesh design for delivery of autologous mesenchymal stem cells influences mesh integration, exposure and biocompatibility in an ovine model of pelvic organ prolapse. Biomaterials. 2019;225:119495. https://doi.org/10.1016/j.biomaterials.2019.119495.
Doucède G, Morch A, Pouseele B, et al. Evolution of the Mechanical Properties of a mechanical properties of a medical device regarding implantation time. Eur J Obstet Gynecol Reprod Biol. 2019;242:139–43. https://doi.org/10.1016/j.ejogrb.2019.08.021.
Ai F-F, Mao M, Zhang Y, Kang J, Zhu L. The in vivo biocompatibility of titanized polypropylene lightweight mesh is superior to that of conventional polypropylene mesh. Neurourol Urodyn. 2020;39:96–107. https://doi.org/10.1002/nau.24159.
Knight KM, Artsen AM, Routzong MR, King GE, Abramowitch SD, Moalli PA. New Zealand white rabbit: a novel model for prolapse mesh implantation via a lumbar colpopexy. Int Urogynecol J. 2020;31:91–9. https://doi.org/10.1007/s00192-019-04071-z.
Paul K, Darzi S, McPhee G, et al. 3D bioprinted endometrial stem cells on melt electrospun poly ε-caprolactone mesh for pelvic floor application promote anti-inflammatory responses in mice. Acta Biomater. 2019;97:162–76. https://doi.org/10.1016/j.actbio.2019.08.003.
Shaffer RM, Liang R, Knight K, Carter-Brooks CM, Abramowitch S, Moalli PA. Impact of polypropylene prolapse mesh on vaginal smooth muscle in rhesus macaque. Am J Obstet Gynecol. 2019;221:330.e1–9. https://doi.org/10.1016/j.ajog.2019.05.008.
Ai F-F, Mao M, Zhang Y, Kang J, Zhu L. Experimental study of a new original mesh developed for pelvic floor reconstructive surgery. Int Urogynecol J. 2020;31:79–89. https://doi.org/10.1007/s00192-019-03947-4.
Hansen SG, Taskin MB, Chen M, Wogensen L, Vinge Nygaard J, Axelsen SM. Electrospun nanofiber mesh with fibroblast growth factor and stem cells for pelvic floor repair. J Biomed Mater Res B Appl Biomater. 2020;108:48–55. https://doi.org/10.1002/jbm.b.34364.
Mukherjee S, Darzi S, Rosamilia A, et al. Blended nanostructured degradable mesh with endometrial mesenchymal stem cells promotes tissue integration and anti-inflammatory response in vivo for pelvic floor application. Biomacromolecules. 2019;20:454–68. https://doi.org/10.1021/acs.biomac.8b01661.
Hympanova L, Mori da Cunha MGMC, Rynkevic R, et al. Experimental reconstruction of an abdominal wall defect with electrospun polycaprolactone-ureidopyrimidinone mesh conserves compliance yet may have insufficient strength. J Mech Behav Biomed Mater. 2018;88:431–41. https://doi.org/10.1016/j.jmbbm.2018.08.026.
Hympánová L, Rynkevic R, Román S, et al. Assessment of electrospun and ultra-lightweight polypropylene meshes in the sheep model for vaginal surgery. Eur Urol Focus. 2020;6:190–8. https://doi.org/10.1016/j.euf.2018.07.024.
Bronzatto E, Riccetto C. Pro-inflammatory cytokines and metalloproteinase activation in polypropylene mesh implant in rat subcutaneous tissue. Int Braz J Urol. 2018;44:819–25.
Lu Y, Fu S, Zhou S, et al. Preparation and biocompatibility evaluation of polypropylene mesh coated with electrospinning membrane for pelvic defects repair. J Mech Behav Biomed Mater. 2018;81:142–8. https://doi.org/10.1016/j.jmbbm.2018.02.030.
Ding J, Han Q, Deng M, et al. Induction of human umbilical cord mesenchymal stem cells into tissue-forming cells in a murine model: implications for pelvic floor reconstruction. Cell Tissue Res. 2018;372:535–47. https://doi.org/10.1007/s00441-017-2781-y.
Iva U, Nikhil S, Geertje C, et al. In vivo documentation of shape and position changes of MRI-visible mesh placed in rectovaginal septum. J Mech Behav Biomed Mater. 2017;75:379–89. https://doi.org/10.1016/j.jmbbm.2017.08.005.
Hympanova L, Mori da Cunha MGMC, Rynkevic R, et al. Physiologic musculofascial compliance following reinforcement with electrospun polycaprolactone-ureidopyrimidinone mesh in a rat model. J Mech Behav Biomed Mater. 2017;74:349–57. https://doi.org/10.1016/j.jmbbm.2017.06.032.
Lu Y, Dong S, Zhang P, Liu X, Wang X. Preparation of a polylactic acid knitting mesh for pelvic floor repair and in vivo evaluation. J Mech Behav Biomed Mater. 2017;74:204–13. https://doi.org/10.1016/j.jmbbm.2017.05.034.
Liang R, Knight K, Easley D, Palcsey S, Abramowitch S, Moalli PA. Towards rebuilding vaginal support utilizing an extracellular matrix bioscaffold. Acta Biomater. 2017;57:324–33. https://doi.org/10.1016/j.actbio.2017.05.015.
Gokmen-Karasu AF, Aydin S, Sonmez FC, Adanir I, Ilhan G, Ates S. A rat hysteropexy model for evaluating adhesion formation and comparison of two different structured meshes. Int Urogynecol J. 2017;28:1695–700. https://doi.org/10.1007/s00192-017-3328-1.
Glindtvad C, Chen M, Vinge Nygaard J, et al. Electrospun biodegradable microfibers induce new collagen formation in a rat abdominal wall defect model: a possible treatment for pelvic floor repair? J Biomed Mater Res B Appl Biomater. 2018;106:680–8. https://doi.org/10.1002/jbm.b.33875.
Bigozzi MA, Provenzano S, Maeda F, Palma P, Riccetto C. In vivo biomechanical properties of heavy versus light weight monofilament polypropylene meshes. Does the knitting pattern matter?. Neurourol Urodyn. 2017;36:73–9. https://doi.org/10.1002/nau.22890.
Avila OR, Parizzi NG, Souza APM, Botini DS, Alves JY, Almeida SHM. Histological response to platelet-rich plasma added to polypropylene mesh implemented in rabbits. Int Braz J Urol. 2016;42:993–8.
Sabiniano R, Iva U, Geertje C, et al. Evaluating alternative materials for the treatment of stress urinary incontinence and pelvic organ prolapse: a comparison of the in vivo response to meshes implanted in rabbits. J Urol. 2016;196:261–9.https://doi.org/10.1016/j.juro.2016.02.067.
Alcalay M, Livneh M, Braun NM, Tov YS, Hod E. Mesh pullout force: comparative study of different deployment techniques in a sheep model. Int Urogynecol J. 2014;25:103–7. https://doi.org/10.1007/s00192-013-2162-3.
Cunha GR, Sinclair A, Ricke WA, Robboy SJ, Cao M, Baskin LS. Reproductive tract biology: of mice and men. Differentiation. 2019;110:49–63. https://doi.org/10.1016/j.diff.2019.07.004.
Otto LN, Slayden OD, Clark AL, Brenner RM. The rhesus macaque as an animal model for pelvic organ prolapse. Am J Obstet Gynecol. 2002;186:416–21. https://doi.org/10.1067/mob.2002.121723.
Jackson R, Hilson RPN, Roe AR, Perkins N, Heuer C, West DM. Epidemiology of vaginal prolapse in mixed-age ewes in New Zealand. N Z Vet J. 2014;62:328–37. https://doi.org/10.1080/00480169.2014.925788.
McLean JW. Vaginal prolapse in sheep. N Z Vet J. 1956;4:38–55. https://doi.org/10.1080/00480169.1956.33219.
Young N, Rosamilia A, Arkwright J, et al. Vaginal wall weakness in parous ewes: a potential preclinical model of pelvic organ prolapse. Int Urogynecol J. 2017;28:999–1004. https://doi.org/10.1007/s00192-016-3206-2.
Mansoor A, Curinier S, Campagne-Loiseau S, Platteeuw L, Jacquetin B, Rabischong B. Development of an ovine model for training in vaginal surgery for pelvic organ prolapse. Int Urogynecol J. 2017;28:1595–7. https://doi.org/10.1007/s00192-017-3292-9.
Chen J, Yu J, Morse A, et al. Effectiveness of self-cut vs mesh-kit titanium-coated polypropylene mesh for transvaginal treatment of severe pelvic organ prolapse: a multicenter randomized noninferiority clinical trial. JAMA Netw Open. 2022;5:e2231869. https://doi.org/10.1001/jamanetworkopen.2022.31869.
Abramowitch SD, Feola A, Jallah Z, Moalli PA. Tissue mechanics, animal models, and pelvic organ prolapse: a review. Eur J Obstet Gynecol Reprod Biol. 2009;144:S146–58. https://doi.org/10.1016/j.ejogrb.2009.02.022.
Mori da Cunha MGMC, Mackova K, Hympanova LH, Bortolini MAT, Deprest J. Animal models for pelvic organ prolapse: systematic review. Int Urogynecol J. 2021;32:1331–44. https://doi.org/10.1007/s00192-020-04638-1.
Fang F, Zhao Z, Xiao J, Wen J, Wu J, Miao Y. Current practice in animal models for pelvic floor dysfunction. Int Urogynecol J. 2023;34:797–808. https://doi.org/10.1007/s00192-022-05387-z.
Flecknell P. Replacement, reduction and refinement. ALTEX. 2002;19:73–8.
Couri BM, Lenis AT, Borazjani A, Paraiso MFR, Damaser MS. Animal models of female pelvic organ prolapse: lessons learned. Expert Rev Obstet Gynecol. 2012;7:249–60.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Amelia Seifalian: conceptualisation, writing, original draft preparation, data collection and data analysis; Amelia Seifalian, Alex Digesu and Vikram Khullar: writing, reviewing and editing; Vikram Khullar: supervision; Amelia Seifalian: project administration. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Conflicts of interest
None.
Additional information
Handling Editor: Tamara Serdinšek
Editor in Chief: Kaven Baessler
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A.S. has been awarded the Young Innovator Next Steps Award 2023 from Innovate UK for the development of a new pelvic membrane to treat pelvic organ prolapse
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seifalian, A., Digesu, A. & Khullar, V. The use of animal models in preclinical investigations for the development of a surgical mesh for pelvic organ prolapse. Int Urogynecol J 35, 741–758 (2024). https://doi.org/10.1007/s00192-024-05741-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-024-05741-3