Abstract
Introduction and hypothesis
We aimed to explore the cellular properties of fibroblasts and smooth muscle cells (SMCs), the two major cell types of the vagina wall, in pelvic organ prolapse (POP) to improve the knowledge of the underlying molecular mechanisms of POP.
Methods
The single-cell RNA sequencing (scRNA-seq) profile GSE151202 was downloaded from NCBI Gene Expression Omnibus, in which vaginal wall tissues were harvested from patients with anterior vaginal wall prolapse and control subjects respectively. The scRNA-seq data of samples (5 POP and 5 controls) were adopted for analysis. Cluster analysis was performed to identify the cell subclusters. Trajectory analysis was applied to construct the differentiation trajectories of fibroblasts and SMCs. Cellular communication analysis was carried out to explore the ligand–receptor interactions between fibroblasts/SMCs and immune cells.
Results
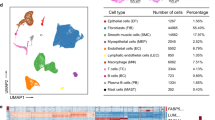
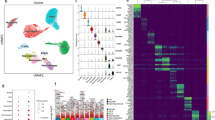
Ten subclusters were determined in both groups, among which fibroblasts and SMCs were the most abundant cell types. Compared with controls, fibroblasts increased whereas SMCs declined in POP. During the transition of fibroblasts and SMCs from a normal into a disease state, extracellular matrix organization and antigen presentation were heightened. The intercellular communications were altered in POP. Interactions between fibroblasts/SMCs and macrophages/natural killer/T cells were strengthened as more ligand–receptor pairs involved in antigen presentation pathways were gained in POP.
Conclusion
Extracellular matrix organization and antigen presentation abilities of fibroblasts and SMCs were enhanced in POP.




Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Funding: The project was supported by Natural Science Research Funds of Minhang District, Shanghai ( No.2022MHZ034).
References
Akeel NY, Gurland B, Hull T. Pelvic floor disorders related to urology and gynecology. Fundamentals of anorectal surgery. Cham: Springer; 2019. p. 571–82.
Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369:1027–38.
Iglesia C, Smithling KR. Pelvic organ prolapse. Am Fam Physician. 2017;96(3):179–85.
Barber MD, Maher C. Epidemiology and outcome assessment of pelvic organ prolapse. Int Urogynecol J. 2013;24(11):1783–90.
Weintraub AY, Glinter H, Marcus-Braun N. Narrative review of the epidemiology, diagnosis and pathophysiology of pelvic organ prolapse. Int Braz J Urol. 2019;46:5–14.
Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in US women: 2010 to 2050. Obstet Gynecol. 2009;114(6):1278–83.
Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186(6):1160–6.
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89(4):501–6.
Chow D, Rodríguez LV. Epidemiology and prevalence of pelvic organ prolapse. Curr Opin Urol. 2013;23(4):293–8.
Kolodziejczyk AA, Kim JK, Svensson V, Marioni JC, Teichmann SA. The technology and biology of single-cell RNA sequencing. Mol Cell. 2015;58(4):610–20.
Li Y, Zhang Q-Y, Sun B-F, et al. Single-cell transcriptome profiling of the vaginal wall in women with severe anterior vaginal prolapse. Nat Commun. 2021;12(1):1–13.
Clark GL, Pokutta-Paskaleva AP, Lawrence DJ, et al. Smooth muscle regional contribution to vaginal wall function. Interface Focus. 2019;9(4):20190025.
De Landsheere L, Munaut C, Nusgens B, et al. Histology of the vaginal wall in women with pelvic organ prolapse: a literature review. Int Urogynecol J. 2013;24:2011–20.
Mangiola S, Doyle MA, Papenfuss AT. Interfacing Seurat with the R tidy universe. Bioinformatics. 2021;37(22):4100–7.
Korsunsky I, Millard N, Fan J, et al. Fast, sensitive and accurate integration of single-cell data with harmony. Nat Methods. 2019;16(12):1289–96.
McGinnis CS, Murrow LM, Gartner ZJ. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 2019;8(4):329–37.e4.
Qiu X, Mao Q, Tang Y, et al. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods. 2017;14(10):979–82.
Jin S, Guerrero-Juarez CF, Zhang L, et al. Inference and analysis of cell-cell communication using CellChat. Nat Commun. 2021;12(1):1–20.
Badiou W, Granier G, Bousquet P-J, Monrozies X, Mares P, de Tayrac R. Comparative histological analysis of anterior vaginal wall in women with pelvic organ prolapse or control subjects. Pilot Study Int Urogynecol J. 2008;19(5):723–9.
Vetuschi A, D’Alfonso A, Sferra R, et al. Changes in muscularis propria of anterior vaginal wall in women with pelvic organ prolapse. Eur J Histochem. 2016;60(1):2604.
Boreham MK, Wai CY, Miller RT, Schaffer JI, Word RA. Morphometric analysis of smooth muscle in the anterior vaginal wall of women with pelvic organ prolapse. Am J Obstet Gynecol. 2002;187(1):56–63.
Yucel N, Usta A, Guzin K, et al. Immunohistochemical analysis of connective tissue in patients with pelvic organ prolapse. J Mol Histol. 2013;44(1):97–102.
Yu Q, Vazquez R, Zabadi S, Watson RR, Larson DF. T-lymphocytes mediate left ventricular fibrillar collagen cross-linking and diastolic dysfunction in mice. Matrix Biol. 2010;29(6):511–8.
Author information
Authors and Affiliations
Contributions
Weimin Fan and DuanQing Wu: concepts, manuscript preparation, manuscript review; Liwen Zhang and Jun Ye: design, data acquisition; Junhua Guan and Ying Yang: data acquisition, data analysis; Xiaohui Mei and Rujun Chen: manuscript editing, manuscript review.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publication
Not applicable.
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Weimin Fan and Duanqing Wu should be regarded as co-first authors.
Xiaohui Mei and Rujun Chen are co-corresponding authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fan, W., Wu, D., Zhang, L. et al. Single-cell transcriptomic data reveal the increase in extracellular matrix organization and antigen presentation abilities of fibroblasts and smooth muscle cells in patients with pelvic organ prolapse. Int Urogynecol J 34, 2529–2537 (2023). https://doi.org/10.1007/s00192-023-05539-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-023-05539-9