Abstract
Introduction and hypothesis
In randomized trials both percutaneous tibial nerve stimulation (PTNS) and sham result in clinically significant improvements in accidental bowel leakage (ABL). We aimed to identify subgroups who may preferentially benefit from PTNS in women enrolled in a multicenter randomized trial.
Methods
This planned secondary analysis explored factors associated with success for PTNS vs sham using various definitions: treatment responder using three cutoff points for St. Mark’s score (≥3-, ≥4-, and ≥5-point reduction); Patient Global Impression of Improvement (PGI-I) of ≥ much better; and ≥50% reduction in fecal incontinence episodes (FIEs). Backward logistic regression models were generated using elements with significance of p<0.2 for each definition and interaction terms assessed differential effects of PTNS vs sham.
Results
Of 166 women randomized, 160 provided data for at least one success definition. Overall, success rates were 65% (102 out of 158), 57% (90 out of 158), and 46% (73 out of 158) for ≥3-, ≥4-, and ≥5-point St Mark’s reduction respectively; 43% (68 out of 157) for PGI-I; and 48% (70 out of 145) for ≥50% FIEs. Of those providing data for all definitions of success, 77% (109 out of 142) met one success criterion, 43% (61 out of 142) two, and 29% (41 out of 142) all three success criteria. No reliable or consistent factors were associated with improved outcomes with PTNS over sham regardless of definition.
Conclusions
Despite exploring diverse success outcomes, no subgroups of women with ABL differentially responded to PTNS over sham. Success results varied widely across subjective and objective definitions. Further investigation of ABL treatment success definitions that consistently and accurately capture patient symptom burden and improvement are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accidental bowel leakage (ABL), also known as fecal incontinence, is defined as the involuntary loss of solid or liquid stool from the rectum [1, 2]. Prevalence estimates range from 7 to 20% among community-dwelling adult women, and rates reach up to 70% in nursing home populations [1,2,3]. The negative impact on psychosocial functioning and quality of life can be devastating, leading to stigmatization, low self-esteem, social isolation, and psychiatric disorders [4, 5]. When conservative options such as lifestyle modifications, pharmacological therapy, and pelvic floor exercises with or without biofeedback are insufficient to control symptoms, more invasive treatments such as vaginal devices, anal plugs, sphincteroplasty, and neuromodulation are considered. Sacral neuromodulation (SNM), currently the only approved neuromodulation modality in the USA for the treatment of FI, is a safe and effective option for refractory FI [2, 6]. However, it is invasive, expensive, and associated with surgical morbidities [7]. Therefore, percutaneous tibial nerve stimulation (PTNS), an approved therapy for urge urinary incontinence, gained interest as a potential minimally invasive office therapy with lower associated morbidities and cost [8].
Two multi-center randomized trials of PTNS have demonstrated no significant difference in primary ABL outcomes compared with sham treatment, although a significant number of participants improved in each treatment arm [9, 10]. Subsequent post hoc analyses of the CONTrol of Faecal Incontinence using Distal NeuromodulaTion (CONFIDeNT) trial demonstrated significant response to PTNS in a subset of participants without obstructive defecation symptoms[11], suggesting PTNS as a potential treatment for select women. The NeurOmodulatTion for Accidental Bowel Leakage (NOTABLe) trial also found no significant difference in improvement in ABL based on the change in St. Mark’s score after 12 weeks of treatment between those randomized to PTNS vs sham [10]. The objective of this planned secondary analysis was to identify potential subgroups who may experience significant treatment success for PTNS over sham treatment.
Materials and methods
The study methods and primary results of the NOTABLe trial have been published [10, 12]. The primary study was IRB approved, with a formal Data and Safety Monitoring Board, and was conducted under a single institutional review board approval by the University of Pittsburgh (NCT 03278613). All participants provided written informed consent.
Eligible women 18 years of age or older with at least 3 months of ABL symptoms and a St. Mark’s score of 12 or greater were randomized 2:1 to either PTNS or sham treatment. A 4-week run-in was conducted to exclude those who reported symptom reduction below the inclusion threshold in response to completing bowel diaries and to self-imposed dietary and behavioral measures alone. Baseline measures included participant demographics, clinical characteristics, medical history and condition-specific ABL severity measures prior to run-in. Baseline quality of life measures were collected after run-in, prior to study-treatment initiation. Initial analyses for the primary study outcome of treatment response (i.e., treatment success) were performed using ≥4-point reduction from baseline in St. Mark’s score. Because published minimum important differences are reported to range from 3- to 5-point reductions in St. Mark’s score [13], treatment response success rates were also explored for ≥3 and ≥5 reductions. We further sought to determine whether additional subjective and objective measures provided consistent results. Thus, we examined success definitions using the Patient Global Impression of Improvement (PGI-I) of very much or much better, and using fecal incontinence episodes (FIEs), of ≥50% reduction from baseline as reported on a 14-day Bowel eDiary [14]. All measures were recorded after 12 weekly sessions of therapy.
Baseline demographic, clinical, and symptom characteristics were compared between treatment success and failure for each of the above definitions. Baseline symptom severity was established prior to the 4-week run-in and was defined as baseline St. Mark’s score for the primary treatment responder outcome, baseline patient global symptom control (PGSC) for the PGI-I outcome, and baseline average FIE per week for the FIE success outcome. Diet was assessed by measuring dietary and supplemental fiber intake as well as a 15-item meat/snack questionnaire that assesses intake of dietary fat (scores ranging from 0 to 60 with higher scores indicating greater dietary fat intake) [15]. Additional baseline bowel diary data included number of bowel movements per week, bowel movements with urgency per week, accident-free days per week, leaks per week, and leaks with urgency per week. Each factor was assessed separately for each success outcome (St. Mark’s reduction of ≥3, 4, and 5; PGI-I and ≥50% FIE reduction at 12 weeks).
Descriptive statistics were used to evaluate success rates for each of the above subjective and objective definitions of success. Given differing rates of success by definition, factors associated with success for each definition were explored using bivariate analyses with Chi-squared tests, Student’s t tests, and Wilcoxon rank-sum tests as appropriate to identify potential demographic and clinical characteristics associated with treatment effect. Given the substantial improvements observed in the sham treatment group in NOTABLe [10], all treatment responders regardless of treatment allocation (i.e., PTNS or sham) were grouped to identify factors associated with any ABL intervention. Subgroups of participants who received greater benefit from PTNS than sham were identified by evaluating the statistical interaction between participant characteristics and assigned treatment group in logistic regression models. Initial models included clinical site, assigned treatment group (PTNS or sham), and baseline severity, plus clinical, demographic, and symptom severity variables significant in bivariate comparisons at the p<0.2 level along with their interactions with treatment group. Backward selection was employed for model generation, with candidate variables removed from the model based on changes to Akaike’s Information Criteria (AIC). Collinearity among the potential model variables was assessed. Adjusted odds ratios (AORs) and 95% confidence intervals (CIs) described the associations between patient characteristics and the success outcomes. A 5% two-sided significance level was used for all statistical testing; no data imputation was performed and no adjustments for multiple testing were made. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
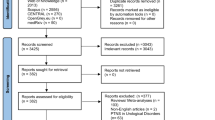
Of the 166 women who completed the 4-week run-in phase and were ultimately randomized to treatment, 162 provided some post-baseline data (108 PTNS, 54 sham) and 160 (96%) had sufficient data after 12 weeks of treatment to determine their status based on at least one of the definitions of success. Complete outcome data, inclusive of St. Mark’s score, PGI-I, and bowel diaries were available for 142 (86%) participants. Among the 160 women included in these analyses, mean age at baseline was 64 (±12) years, with 11% Black, 9% Latina, and 80% white self-identified race and ethnicity, and a mean body mass index (BMI) of 29 (±7 kg/m2) and baseline St. Mark’s score of 18 (±3).
The proportion meeting criteria for success was 65% (102 out of 158: 68 PTNS, 34 sham), 57% (90 out of 158: 64 PTNS, 26 sham), and 46% (73 out of 158: 52 PTNS, 21 sham) for the responder outcome (≥3-, 4-, and 5-point reduction from baseline in St. Mark’s score respectively), 43% (68 out of 157: 47 PTNS, 21 sham) for the PGI-I, and 48% (70 out of 145: 51 PTNS, 19 sham) based on the diary variable of ≥50% reduction in FIEs. We also assessed overlaps in success definitions among the 142 women who provided data for all definitions of success. When defining the responder status as ≥4-point change from baseline in St. Mark’s (the NOTABLe primary study outcome), 77% (109 out of 142) met 1 or more success criteria, 43% (61 out of 142) met 2 criteria, and 29% (41 out of 142) met all 3 success criteria. There was no marked change in concordance when the St. Mark’s threshold was changed. Using responder status as ≥3-point reduction in St. Mark’s score, 80% (114 out of 142) met 1 or more success criterion, 46% (65 out of 142) met 2 criteria, and 30% (42 out of 142) met all 3 success criteria. Meanwhile, using responder status as ≥5-point reduction in St. Mark’s score, 70% (100 out of 142) met 1 or more success criteria, 40% (57 out of 142) met 2 criteria, and 27% (39 out of 142) met all 3 success criteria. Figure 1 demonstrates the overlap in various success outcomes within this population using each of the St. Mark’s minimally important difference cutoff points. Of the 83 women with available data for all success definitions and who met criteria for treatment response in the St. Mark’s questionnaire (58% of the total), only 50 (35% of the total) responded that their symptoms were much or very much better. Additionally, there were 41 women (29% of the total) who had success according to St. Mark’s or PGI-I criteria but did not have ≥50% reductions in FIE. The largest discordance was observed among individuals who were classified as treatment responders but did not have a ≥50% reduction in FIE or did not report themselves to be much or very much better on the PGI-I (n=26), regardless of the St. Mark’s score cutoff point utilized to define treatment response.
Baseline demographic, clinical, and incontinence severity characteristics of women with vs without success (based on response of ≥4-point reduction in St. Mark’s score, PGI-I, and ≥50% reduction in FIEs) had minimal overlap. Supplemental Tables 1 and 2 provide baseline characteristic comparisons between responders and nonresponders for each definition. Results of the multivariate logistic regression models with evidence of treatment effects are included in Table 1, using the St. Mark’s reduction ≥4 along with PGI-I and ≥50% improvement in FIEs.
For the outcome of responders based on St. Mark’s score, the only statistically significant interaction effects between treatment groups were noted for BMI and previous urinary incontinence (UI) surgery. In these interactions, women with normal or underweight BMI (<25 kg/m2) or with previous UI surgery were more likely to be treatment responders when receiving PTNS than sham (BMI: AOR 16.61 (95% CI 2.20, 125.18), p<0.01; previous UI surgery: AOR 6.76 (95% CI 1.10 41.30), p<0.05).
For PGI-I, interactions were observed between treatment group and both meat/snack score and education. Women reporting lowest fat intake (lowest tertile on the meat/snack scores) who were randomized to PTNS were more likely than the sham group to express an impression of improvement (AOR 4.25 (95% CI 1.25, 14.45), p<0.05). Further examination of the interaction with education did not reveal greater benefit from PTNS versus sham for any education subgroup.
For objective success of ≥50% improvement in FIEs on the 14-day bowel diary, there were interaction effects between treatment group and both fiber supplementation and baseline FIE frequency. Although overall success was lower in those women on fiber supplementation, those on fiber supplements who received PTNS were more likely to experience objective success than those who received sham (AOR 15.20 [95% CI 1.95, 118.35], p<0.01); however, this was driven primarily by a lack of response in the sham group. Additionally, participants with higher baseline FIE frequency were more likely to experience objective success in the PTNS group than in the sham group (AOR 5.34 [95% CI 1.19, 23.96], p<0.05).
Given the observed associations with dietary factors of fiber and fat intake, a supplemental analysis was conducted that examined the relationship between baseline fiber supplement use and stool consistency, as measured by the Bristol Stool Scale. No association between fiber supplement use and the Bristol Stool Scale score was observed (data not shown).
There were no significant treatment effects for PTNS for success definitions using St. Mark’s reduction of ≥3 or ≥5 (Supplemental Table 3).
Discussion
The NOTABLe trial is the second multicenter, randomized, masked trial of PTNS for treatment of ABL to report no benefit over sham stimulation [9, 10]. In this planned secondary analysis, we aimed to determine whether patient characteristics were associated with PTNS treatment benefit in order to potentially identify a subgroup of patients who may benefit from peripheral neuromodulation. No consistent predictors of response to treatment were identified in this analysis across multiple definitions of success using both subjective and objective criteria. Furthermore, in reviewing these data, we found that success rates varied widely according to the success definition used, with only 29% meeting success according to all three of the analyzed criteria based on St. Mark’s ≥4-point improvement, PGI-I, and reductions in FIE, whereas 77% of the study population met at least one of the success definitions.
This heterogeneity in outcome status is notable, as it highlights the importance of the definition utilized when assessing ABL treatment efficacy and suggests potential variability in standardized research trials compared with common clinical intervention. Indeed, prior research of pelvic floor dysfunction has revealed discordance in the patient assessment of treatment success compared with objective measures with respect to surgical treatment of pelvic organ prolapse [16, 17]. However, unlike prior studies that showed a strong correlation of subjective impressions of patient improvement, even when discordant from objective anatomical measures, our data suggest that, among women with refractory ABL, there remains substantial discordance in patient assessment of treatment success, with less than one third of women reporting subjective success without evidence of objective success. Given that objective outcomes are typically required for permanent implantation of a sacral neuromodulation device, these findings further highlight the potential weakness of our existing disease severity measures. Moreover, the optimal cutoff point for the St. Mark’s score minimally important differences is not universally accepted and ranges from 3 to 5 [13]. In this cohort of women, the variation in cutoff point definition resulted in a variation in success rates of nearly 20%. Additionally, using the 3- and 5-point thresholds revealed no consistent treatment effects between PTNS and sham and did not improve concordance in the success definitions. Based on these findings, we suggest further investigation into the optimal definitions of success and failure for ABL intervention trials.
With respect to potential differential efficacy of PTNS vs sham, although there were isolated risk factors that met the significance criteria in our analyses, they were not consistent across our outcome measures or differential response thresholds. As such, our study failed to identify clinically meaningful differences in the application of PTNS to any subgroup of women with ABL. Notably, however, this analysis was exploratory in nature, did not include qualitative assessments and the study was not designed or powered to find differences in response between subgroups. Additionally, this study did not perform sophisticated characterization of anal sphincter defects, rectal tone, and compliance or pudendal neuropathy. Though not part of the NOTABLe study design, high-resolution anorectal manometry with rectal sensory and compliance testing may have helped to characterize individuals with affected rectal tone and compliance who may have differing responses to treatment. Additionally, NOTABLe did not ascertain through questionnaire or structured interview baseline obstructive defecation (OD) symptoms of difficulty with rectal evacuation, excessive straining, requirement for regular digitation, or a sensation of incomplete emptying. As such, we cannot support or refute the negative association between OD and PTNS treatment outcomes identified in a post-hoc subgroup analysis of the CONFIDeNT trial [11].
Our study findings are strengthened by the use of a large number of participants with uniform, validated data collection from a multicenter, randomized trial. However, given the inclusion criteria applied to the NOTABLe trial, the results are not generalizable to men or to individuals with less-severe ABL undergoing behavioral or pharmacological treatment. Fecal incontinence is known to have a significant impact on patient wellbeing and quality of life, but those influences are multifactorial and individualized [18]. Given the differential outcome experiences, as well as the heterogeneity in success outcomes demonstrated here and in other intervention studies [19], our findings further emphasize the importance of careful counseling to assess patient bother, as well as treatment goals and expectations. Further evaluation of existing novel outcome measures, including the Accident Bowel Leakage Evaluation [20] instrument as a potential outcome measure of treatment response, may help in the quest for the appropriate outcome assessment for ABL treatments.
Conclusion
Our data reinforce prior findings and fail to find consistent predictors of response to PTNS for the treatment of women with refractory ABL. However, these data do suggest that there remains substantial discordance in outcomes across the various current measures of fecal incontinence. Further assessment of optimal outcomes for ABL treatment is needed. These findings may subsequently benefit future investigations of interventions for ABL.
References
Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: State of the Science Summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127–36.
Dunivan GC, Chen CCG, Rogers R. ACOG Practice Bulletin No. 210: fecal incontinence. Obstet Gynecol. 2019;133:E260–73. https://doi.org/10.1097/AOG.0000000000003187.
Brown HWW, Wexner SDD, Segall MMM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int J Clin Pract. 2012;66:1101–8. https://doi.org/10.1111/ijcp.12018.
Crowell MD, Schettler VA, Lacy BE, et al. Impact of anal incontinence on psychosocial function and health-related quality of life. Dig Dis Sci. 2007;52:1627–31. https://doi.org/10.1007/s10620-006-9249-3.
Brown HW, Wexner SD, Segall MM, et al. Quality of life impact in women with accidental bowel leakage. Int J Clin Pract. 2012;66:1109–16. https://doi.org/10.1111/ijcp.12017.
Paquette IM, Varma MG, Kaiser AM, et al. The American Society of Colon and Rectal Surgeons’ clinical practice guideline for the treatment of fecal incontinence. Dis Colon Rectum. 2015;58:623–36.
Thin NN, Horrocks EJ, Hotouras A, et al. Systematic review of the clinical effectiveness of neuromodulation in the treatment of faecal incontinence. Br J Surg. 2013;100:1430–47. https://doi.org/10.1002/bjs.9226.
Martinson M, MacDiarmid S, Black E. Cost of neuromodulation therapies for overactive bladder: percutaneous tibial nerve stimulation versus sacral nerve stimulation. J Urol. 2013;189:210–6. https://doi.org/10.1016/j.juro.2012.08.085.
Knowles CH, Horrocks EJ, Bremner SA, et al. Percutaneous tibial nerve stimulation versus sham electrical stimulation for the treatment of faecal incontinence in adults (CONFIDeNT): a double-blind, multicentre, pragmatic, parallel-group, randomised controlled trial. Lancet. 2015;386:1640–8. https://doi.org/10.1016/S0140-6736(15)60314-2.
Zyczynski HM, Richter HE, Sung VW, et al. Percutaneous tibial nerve stimulation vs sham stimulation for fecal incontinence in women: neuromodulation for accidental bowel leakage randomized clinical trial. Am J Gastroenterol. 2022;117:654–67. https://doi.org/10.14309/ajg.0000000000001605.
Horrocks EJ, Chadi SA, Stevens NJ, et al. Factors associated with efficacy of percutaneous tibial nerve stimulation for fecal incontinence, based on post-hoc analysis of data from a randomized trial. Clin Gastroenterol Hepatol. 2017;15:1915–1921.e2. https://doi.org/10.1016/j.cgh.2017.06.032.
Zyczynski HM, Arya LA, Lukacz ES, et al. Design of a randomized controlled trial of percutaneous posterior tibial nerve stimulation for the treatment of refractory fecal incontinence in women: the neuromodulation for accidental bowel leakage study. Female Pelvic Med Reconstr Surg. 2021;27:726–34. https://doi.org/10.1097/SPV.0000000000001050.
Bols EMJ, Hendriks EJM, Deutekom M, et al. Inconclusive psychometric properties of the Vaizey score in fecally incontinent patients: a prospective cohort study. Neurourol Urodyn. 2010;29:370–7. https://doi.org/10.1002/nau.20758.
Zyczynski HM, Richter HE, Sung VW, et al. Performance, acceptability, and validation of a phone application bowel diary. Neurourol Urodyn. 2020;39:2480–9. https://doi.org/10.1002/nau.24520.
Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000;18:284–8. https://doi.org/10.1016/S0749-3797(00)00119-7.
Barber MD, Brubaker L, Nygaard I, et al. Defining success after surgery for pelvic organ prolapse. Obstet Gynecol. 2009;114:600–9. https://doi.org/10.1097/AOG.0b013e3181b2b1ae.
Jelovsek JE, Gantz MG, Lukacz E, et al. Success and failure are dynamic, recurrent event states after surgical treatment for pelvic organ prolapse. Am J Obstet Gynecol, 2021;224:362.e1–11
Meyer I, Richter HE. Impact of fecal incontinence and its treatment on quality of life in women. Women Health. 2015;11:225–38.
Richter HE, Jelovsek JE, Iyer P, et al. Characteristics associated with clinically important treatment responses in women undergoing nonsurgical therapy for fecal incontinence. Am J Gastroenterol. 2020;115:115–27. https://doi.org/10.14309/ajg.0000000000000482.
Rogers RG, Bann CM, Barber MD, et al. The responsiveness and minimally important difference for the Accidental Bowel Leakage Evaluation questionnaire. Int Urogynecol J. 2020;31:2499–505. https://doi.org/10.1007/s00192-020-04367-5.
Acknowledgements
We would like to acknowledge the Pelvic Floor Disorders Network (PFDN) and the NOTABLe protocol development team members for their contributions in designing, implementing, and completing the trial: Halina M. Zyczynski, MD; Holly E. Richter, MD; Vivian W. Sung, MD; Emily S. Lukacz, MD; Lily A. Arya, MD; David D. Rahn, MD; Anthony G. Visco MD; Donna Mazloomdoost, MD; Benjamin Carper; PhD; Marie G. Gantz, PhD. This research was funded through the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health at National Institutes of Health.
Financial support
Eunice Kennedy Shriver National Institute of Child Health and Human Development and the NIH Office of Research on Women’s Health at National Institutes of Health Grants: 2UG1 HD069006, 2UG1 HD041261, 2UG1 HD069013, 2 UG1 HD069010, 2 UG1 HD054214, 2 UG1 HD041267, 2 UG1 HD054241, 2 U24 HD069031.
Author information
Authors and Affiliations
Consortia
Contributions
D. Luchristt: data analysis, manuscript writing and editing; B. Carper: data management, data analysis, manuscript writing and editing; S. Balgobin: manuscript writing and editing; I. Meyer: manuscript writing and editing; D. Myers: manuscript writing and editing; D. Mazloomdoost: manuscript writing and editing; M. Gantz: project development, data management, data analysis, manuscript writing and editing; U. Andy: manuscript writing and editing; H. Zyczynski: project development, data analysis, manuscript writing and editing; E.S. Lukacz: project development, data analysis, manuscript writing and editing.
Corresponding author
Ethics declarations
Ethics
Secondary analysis of data from a trial conducted at 9 US clinical sites of the National Institutes of Health Pelvic Floor Disorders Network under the approval of a Data and Safety Monitoring Board and the University of Pittsburgh institutional review board (NCT 03278613).
Conflicts of interest
E.S. Lukacz reports consulting for Axonics. None of the other authors has any conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luchristt, D., Carper, B., Balgobin, S. et al. Characteristics associated with subjective and objective measures of treatment success in women undergoing percutaneous tibial nerve stimulation vs sham for accidental bowel leakage. Int Urogynecol J 34, 1715–1723 (2023). https://doi.org/10.1007/s00192-022-05431-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05431-y