Abstract
Introduction and hypothesis
Urinary incontinence following a pelvic floor muscle (PFM) dysfunction is a common disorder in women with multiple sclerosis (MS). Concurrent anodal transcranial direct current stimulation (a-tDCS) of the primary motor cortex (M1) may prime the effects of PFM training (PFMT) in MS patients. This study was aimed at investigating the effects of M1 a-tDCS on the effectiveness of PFMT in the treatment of female MS patients with urinary incontinence and PFM dysfunctions.
Methods
In a randomized double-blinded, control trial study, 30 women with MS were divided into two groups (experimental group: concurrent active M1 a-tDCS and PFMT; control group: concurrent sham M1 a-tDCS and PFMT). Over the course of 8 weeks, these patients received 20-min interventions three times a week. As an indication of PFM function, the bladder base displacement was measured by ultrasonography before, during the 4th week, immediately, and 1 month after the intervention ended. Urinary incontinence was also measured by Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UISF) before, immediately, and 1 month after the intervention ended.
Results
A significant improvement in PFM function occurred in the 4th week of intervention and remained 1 month after the intervention in the experimental group when compared with the control group (p<0.05). Compared with baseline, both groups reported significant improvements in PFM function at 8 weeks (p<0.05). Also, both groups were found to have decreased ICIQ-UIS scores after the intervention and at 1-month follow-up (p<0.05).
Conclusions
In MS patients, M1 a-tDCS can significantly enhance the effects of PFMT on the PFM function and urinary incontinence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is an autoimmune disease, which leads to the demyelination of axons in the central nervous system (CNS), with sensory, motor, and cognitive symptoms [1]. In Europe, the prevalence of MS is estimated at 83 cases per 100,000 people [1]. In Iran, the prevalence of MS is 88 per 100,000 population, which indicates the high risk of this disease in this area [2]. Pelvic floor muscle (PFM) dysfunctions and urinary disorders are the most important motor disorders in patients with MS, which strongly affect their quality of life (QOL) [3].
Urinary disorders and PFM dysfunctions deteriorate with the progression of MS [4]. Although these symptoms are not life threatening in these patients, they mainly reduce the quality of the patient’s work and general QOL [4]. Therefore, management of urinary disorders by improving the function of PFM is critical in patients with MS [5, 6]. Studies suggest that regular application of PFM training (PFMT) for 4–6 weeks may not be sufficient. However, in some studies, persistent application of PFMT for 12 weeks resulted in a modest improvement in the function of PFMs in these patients [6,7,8]. On the other hand, fatigue and reduced motivation to continue long-term training sessions are among the major problems of MS patients during long-term training [5,6,7]. Consequently, it is important to develop an intervention that can improve PFM function, control urinary disorders, and enhance the MS patients’ QOL with high efficacy in the shortest time possible [5,6,7].
Evidence suggests that the proper function of PFMs in MS patients depends on the integrity of both the central and peripheral motor pathways at different levels of the nervous system [8]. Nevertheless, almost all previous studies have focused on improving the peripheral motor nervous system and attempted to directly improve the PFM function through exercise therapy [5, 6, 9]. Generally, MS causes structural and functional changes in different parts of the CNS [8]. Therefore, the use of techniques focusing on the induction of changes in the CNS is necessary for these patients [8].
The primary motor cortex (M1) is one of the key cortical areas, which undergoes structural and functional changes in MS. The modulation of this area may improve the function of PFMs [8]. The use of neuromodulatory techniques, such as anodal transcranial direct current stimulation (a-tDCS) over M1, can enhance the efficacy of conventional peripheral therapies (i.e., PFMT) in the treatment of PFM dysfunctions and urinary disorders in patients with MS [9]. Generally, tDCS is an effective, non-invasive, inexpensive, accessible, and painless cortical stimulation technique [10]. Numerous studies have reported different therapeutic benefits of this technique in MS patients [7, 11, 12]. However, no study has yet investigated the enhancing impact of M1 a-tDCS on the effectiveness of PFMT in PFM function and urinary disorders in MS patients.
With this background in mind, the current study mainly aimed to investigate the short-term and long-term effects of multiple sessions of tDCS on the effectiveness of PFMT in the treatment of female MS patients with urinary incontinence and PFM dysfunctions. It was hypothesized that multiple sessions of concurrent sham M1 a-tDCS and PFMT might improve the PFM function and reduce urinary incontinence in MS patients. Based on the results, the concurrent application of active M1 a-tDCS and PFMT was more effective than multiple sessions of concurrent sham a-tDCS and PFMT in improving the PFM function and reducing urinary incontinence.
Materials and methods
Design
This study had a parallel, double-blind, randomized controlled trial design and was approved by the Human Ethics Committee at Semnan University of Medical Sciences, Semnan, Iran. The study was registered in the Iranian Registry of Clinical Trials (www.irct.ir; IRCT20201102049234N1). The recruitment and follow-up dates of the study were from 14 November 2020 to 21 April 2021. The study data were also collected in the Neuromuscular Rehabilitation Research Center of Semnan University of Medical Sciences. A consent form was signed by all participants, before participating in the study.
Participants
Forty-eight patients with MS were recruited from a neurological clinic and screened based on the inclusion and exclusion criteria by a neurologist. Eighteen patients were excluded according to the following criteria: an Expanded Disability Status Scale (EDSS) score >6.5 (n=2); not being in a stable phase of MS (n=1); a lack of urinary incontinence symptoms (n=3); and an unwillingness to participate in the study because of the COVID-19 pandemic (n=12). The remaining 30 patients were randomly assigned to one of two groups by a computerized random number generator:
-
1.
Experimental group (n=15), receiving multiple sessions of active M1 a-tDCS concurrently with PFMT
-
2.
Sham group (n=15), receiving multiple sessions of sham M1 a-tDCS and PFMT concurrently
All 30 participants, with a mean age of 38.83±5.43 years, completed this study. The sample size of this pilot study, which was calculated using Cohen’s table (based on the effect size determined by the data from the first 20 patients), allowed for investigating the effects of multiple concurrent sessions of M1 a-tDCS and PFMT at a power of 80%. This statistical table summarizes the relationship between the effect size, degree of freedom, power, and the required sample size.
The inclusion criteria of this study were as follows: female patients in the age range of 18–45 years with definite relapsing-remitting MS (a stable stage of MS) over the past 4 months [6]; receiving stable pharmacological therapies in the past 6 months [13]; having symptoms of urinary incontinence [6]; having the ability to contract the PFMs (based on the manual muscle test [MMT] grade ≥3) [6]; having the cognitive capacity to complete the assessments and treatment protocol [6]; have a willingness to participate in the study; having an EDSS score <6.5 [5, 6]; and having a diagnosis of MS according to the 2010 Revised McDonald criteria [14]. The Persian version of the International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-UISF) was also used to investigate urinary incontinence in the patients included [15, 16].
On the other hand, the exclusion criteria were as follows: history of MS relapse during treatment [6]; being pregnant or being in the first 6 months postpartum [5, 6]; caesarean section or vaginal delivery within the past 6 months before enrolment in the study [6]; pelvic organ prolapse in a vaginal examination [5, 6]; urinary tract infection [6]; a history of surgical treatment for urinary incontinence [5]; a history of physiotherapy for urinary incontinence [5]; any other neurological or renal diseases [5]; contraindications for electrical stimulation [17]; and an inability to understand or comply with the study procedures. Figure 1 presents the flowchart of eligibility assessment throughout the study.
Primary outcome measures
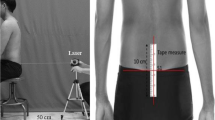
A diagnostic ultrasound imaging unit (HS-2100 V, Japan), set in B-mode with a 7.5-MHz linear head transducer, was used to assess the function of PFMs. Real-time ultrasound imaging is a reliable and valid technique for the assessment of muscle structure, function, and activity [18]. To measure the PFM function, the amount of bladder base uplift was accurately measured [18]. The transducer was placed over the suprapubic area of the lower abdomen in the transverse plane and angled in a caudal/posterior direction (at a 15–30° angle from the vertical direction) to obtain a clear image of the inferior–posterior aspect of the bladder.
To acquire clear images of the bladder base following ultrasound imaging, a standardized bladder-filling protocol was applied. The participants consumed 600–750 ml of water 30 min before the test [18]. By calculating the length and width of the bladder with ultrasound, the bladder volume was measured [19]. The participants were evaluated in a supine crook-lying position, with pillows placed underneath their head, hips, and knees, their knees flexed to approximately 60°, and their lumbar spine positioned in an approximately neutral position. The patients were asked to contract the PFM as hard as they could during the measurements. The amount of bladder base displacement from the resting position to the end of each contraction was measured in millimeters (mm) as an indicator of PFM function [18].
Before the current study, the intra-rater reliability of ultrasound for the measurement of bladder base displacement during PFM contraction was assessed in a test–retest trial of 10 patients with MS. In this reliability analysis, each testing condition was assessed in two separate sessions 3 days apart. During each testing session, each condition was tested three times, and the average value was calculated for further analysis. In the present study, the PFM function was assessed at the onset of the first session (baseline, T0), 4 (T1), and 8 weeks (T2) after the onset of the intervention, and 1 month after the end of the intervention (T3). All measurements were performed by an expert physiotherapist (Fig. 2).
Secondary outcome measures
The participants’ urinary incontinence was assessed by the Persian version of ICIQ-UISF, which includes six questions and is recognized as a valid and reliable tool for the assessment of urinary incontinence. Based on this questionnaire, urinary incontinence was classified into four stages: mild (score, 1–5), moderate (score, 6–12), severe (score, 13–18), and very severe (score, 19–21) [15, 16]. Overall, any reduction in the score of this questionnaire indicates improvement in urinary incontinence. This questionnaire was completed at the beginning of the first session (T0), 8 weeks after the onset of the intervention (T2), and 1 month after the end of the intervention (T3; Fig. 2).
Interventions
Two physiotherapists were involved in performing the interventions. One of the physiotherapists (administrator), who was an expert in instructing PFMT and applying tDCS, carried out the interventions for both groups, and the other therapist (assessor), who was blinded to group assignments, assessed the outcome measures. The participants of both groups were also blind to the nature of the intervention they received. All patients in the two groups participated in three weekly 20-min sessions for 8 weeks.
The experimental group received concurrent active M1 a-tDCS and PFMT, whereas the sham group received concurrent sham a-tDCS and PFMT. All the participants were similarly instructed to conduct progressive Kegel exercises as PFMT [20]. First, a physiotherapist instructed the method of performing the exercises using verbal and visual aids (e.g., photographs) for each group. The therapist supervised all stages of exercise therapy to ensure that the patients performed the exercises correctly. The exercises were progressively ramped up from lying in a supine position to sitting on a Swiss ball [20] as described below:
-
1.
Contraction of PFMs during exhalation by puckering the anus, vagina, and urethra in a supine position, with a backward tilt of the pelvis for 5–10 s [20].
-
2.
Puckering and tightening the PFMs during exhalation in a supine position with a ball under the feet; the ball is pushed away, and the pelvis is lifted during contraction [20].
-
3.
Squeezing the PFMs during the double-leg bridge exercise [20].
-
4.
Gripping the Swiss ball with the sitting bones, contracting the PFMs, and moving forward [20].
-
5.
Rolling the Swiss ball from side to side while being seated, contracting the PFMs, and holding the position for 5–10 s before relaxation at the end of the movement [20].
-
6.
Abducting the hips and contracted PFMs in a sitting position on a Swiss ball and then with a resistive elastic band (Thera-Band) [20].
In this study, an a-tDCS device (ActivaDose® II, ActivaTeK™ Inc., Gilroy, CA, USA) was used to apply a direct, 1.5-mA current over M1 for 20 min. At the onset and end of stimulation, the period of ramp-up and ramp-down was 30 s. To improve the contact of a-tDCS electrodes with the scalp and to reduce resistance, the hair in the area was pulled away as far as possible; the skin of the target area was also cleaned and moisturized.
All the patients participated in 20-min M1 a-tDCS and 20-min PFMT concurrently. In both groups, the anode (5×7 cm2; density, 0.06 mA/cm2), and return (5×7 cm2) electrodes were located over the left M1 and the right contralateral supraorbital area respectively [7]. In the sham group, stimulation was slowly turned off after 30 s of stimulation. The fade-in short stimulation fade-out (FiSsFo) approach was used as a reliable method of maintaining the blinding integrity in the concurrent application of sham a-tDCS and PFMT to induce the assumed initial cutaneous sensations.
Assessment of side effects (or adverse effects)
Any side effects or adverse effects of M1 a-tDCS were monitored in the two groups. The presence and severity of these effects were assessed under electrodes during and after the intervention using a questionnaire with rating and numerical analog scales (e.g., from 0, “no tingling” to 10, “worst tingling imaginable”) [21].
Statistical analysis
The collected data in the current study were analyzed using SPSS Version 24. Shapiro–Wilk test was used to assess the normal distribution of data. The results indicated that all data were normally distributed. Independent t test was also used to assess differences in the baseline values between the groups. Besides, the intra-class correlation (ICC), standard error of measurement (SEM), and minimal detectable changes (MDC) were calculated to investigate the inter- and intra-session reliability of ultrasound measurements of bladder base displacement during PFM contractions. Additionally, general linear mixed model (GLM) repeated measures ANOVA was performed to assess the main effects of group (i.e., concurrent M1 a-tDCS and PFMT and concurrent sham a-tDCS and PFMT) and time (i.e., before, immediately after, and 1 month after the end of the intervention), as well as their interaction effects on both PFM function and urinary incontinence. Moreover, changes in bladder base displacement and the scores of ICIQ-UISF were analyzed within groups using pairwise comparison tests. Type I error (α) was set at 0.05, and the power of tests was considered to be 0.85.
Results
The demographic and baseline data of the participants in each group are presented in Table 1. There was no significant difference between the groups regarding the variables of age, body mass index, and urinary incontinence. Similarly, there was no significant difference in the amount of bladder base displacement from the resting position to the end of each contraction, as a function of PFM, between the groups at baseline (before the interventions; Table 1).
Moreover, the results indicated high intra-session and inter-session reliability for ultrasound measurements of bladder base displacement during PFM contraction in the test and retest sessions. The intra-session ICCs of the test session ranged from 0.96 to 0.98; the ICCs of the retest session ranged from 0.95 to 0.98, and the inter-session ICCs ranged from 0.93 to 0.98. Besides, the SEM and MDC of ultrasound measurements for bladder base displacement from the resting position to the end of each contraction were 0.03 and 0.06 mm respectively.
The results of GLM repeated measures ANOVA indicated the significant main effect of time on the function of PFM and urinary incontinence (p<0.001). The results of ANOVA also indicated a significant group×time interaction effect on urinary incontinence (p=0.002). Besides, a post-hoc analysis was performed using the Bonferroni correction test (Fig. 3). The results indicated a significant increase in the bladder base displacement 4 weeks (T1) and 8 weeks (T2) after the onset of the intervention and also, 1 month after the end of the intervention (T3) in the concurrent active M1 a-tDCS+PFMT group (Fig. 3a). Additionally, the scores of ICIQ-UISF significantly decreased 8 weeks after the onset of the intervention (T2) and also 1 month after the end of the intervention (T3) in the concurrent M1 a-tDCS+PFMT group (Fig. 3b).
a Bladder base displacement during PFM contraction (mean ± SD), T0: first session, T1: 4 weeks after the start of the intervention, T2: 8 weeks after the start of the intervention, T3: 1 month after the end of the intervention. b Urinary incontinence (mean ± SD), T0: first session, T2: 8 weeks after the start of the intervention, T3: 1 month after the end of the intervention
In the sham group (sham a-tDCS+PFMT), a significant increase in the bladder base displacement and a decrease in the score of ICIQ-UISF were observed only 8 weeks (T2) after the onset of the intervention, whereas there was no significant difference in the bladder base displacement 4 weeks after the onset of the intervention (T1) and also, 1 month after the end of the intervention (T3; Fig. 3). Besides, the results of pairwise comparisons showed significant differences in the bladder base displacement (T1 and T3) and the scores of ICIQ-UISF (T3) between the experimental and sham groups (p<0.05; Fig. 3).
The minimal clinically important difference (MCID) of bladder base displacement was estimated to be 0.03 in the current study, based on the distribution method [22]. According to the results, the MCIDs between the groups were 0.08 and 0.15 at T1 and T3 respectively, which exceeded the calculated MCIDs for this outcome. Also, based on the results (Fig. 3b), the MCID between the groups was 4.27 at T3, which exceeded the MCID of the ICIQ-UISF score (score of 1.7) [23]. These findings indicated the clinical efficacy of concurrent active M1 a-tDCS and PFMT in improving the PFM function and urinary incontinence in patients with MS.
All the participants of this study completed the interventions with minimal side effects. Itching was a common side effect of M1 a-tDCS (phases of M1 a-tDCS: beginning, 2.30±1.42; middle, 2.25±1.17; and ending, 1.41±1.20). However, the patients did not report any burning sensation or pain during M1 a-tDCS.
Discussion
This study, for the first time, investigated the impact of multi-session a-tDCS on the effectiveness of PFMT for female patients with MS, who experienced urinary incontinence and PFM dysfunction. The results showed that concurrent multi-session active M1 a-tDCS and PFMT significantly improved the function of PFMs by the 4th and 8th weeks of the intervention and also in the 1-month follow-up. Moreover, the rate of urinary incontinence significantly reduced in both groups after 8 weeks of intervention, whereas the therapeutic effects were only maintained in the active a-tDCS group until the 1-month follow-up.
In the current study, it was hypothesized that concurrent multi-session active M1 a-tDCS and PFMT could improve urinary incontinence and PFM function in patients with MS. The results approved this hypothesis and showed a significant improvement in the PFM function by the 4th week of concurrent active M1 a-tDCS and PFMT, although the treatment period was not completed; also, the positive effects of the intervention were maintained for 1 month. As one of the major problems of MS patients is increased fatigue and reduced motivation to continue the intervention during long-term exercises, combination of tDCS with PFMT can improve the function of PFM, control urinary disorders, and increase the QOL with a high efficacy in the shortest time possible.
In this regard, a systematic meta-analysis by Lattari et al. showed the positive effect of a-tDCS on the muscle function [24]. The results revealed that the use of a-tDCS over the motor cortex area, temporal cortex, and dorsolateral prefrontal cortex (DLPFC) could be useful as an adjunctive treatment to increase the muscle strength, voluntary contraction, and muscle endurance in healthy individuals, as well as strength training athletes and sedentary people [24]. Moreover, Allman et al. assessed the effects of tDCS and selected motor exercises on the motor skills of stroke patients and concluded that tDCS combined with selected motor exercises could enhance the effects of motor exercises and improve the outcomes of long-term rehabilitation in these patients, whereas motor exercises alone could not cause significant changes in their motor skills [25]. MRI also showed an increase in activity and gray matter volume in the premotor and motor cortices of the experimental group following the application of a-tDCS; these improvements persisted for at least 3 months after tDCS [25].
Additionally, Hamoudi et al. examined the effects of tDCS on the stages of motor skill learning in patients with chronic stroke and found significant improvements in their motor skills immediately after the intervention, which confirmed the results of this study. However, in their study, the long-term effects of tDCS were not maintained for 4 months after the intervention [26]. The main cause of this discrepancy between the results of the present study and the study by Hamoudi et al. may be the application of more intense and longer interventions in the present study [26]. Besides, M1 a-tDCS was performed along with PFMT in the current study, whereas tDCS alone was employed in the study by Hamoudi and colleagues. Nevertheless, the higher efficacy of concurrent M1 a-tDCS and PFMT has been reported in the literature [25, 27].
Although no study has yet examined the effects of concurrent M1 a-tDCS and PFMT on the PFM function and urinary incontinence of patients with MS, in a protocol study by Dutra et al., the effects of five sessions of M1 a-tDCS (2-mA, 20-min) on urinary incontinence were assessed using the ICIQ-Female Lower Urinary Tract Symptoms Modules (ICIQ-FLUTS) questionnaire 1 week before the intervention, in the fifth session of the intervention, and 1, 6, and 12 months after the intervention. They found a significant improvement in the urinary incontinence of patients with painful bladder syndrome [28].
Another hypothesis of the present study was that concurrent sham M1 a-tDCS and PFMT did not have any significant positive effects on improving urinary incontinence and PFM function in patients with MS. The current findings showed that the PFM function improved significantly only after an 8-week intervention; no effects were observed before this period. Also, the effects of concurrent sham M1 a-tDCS and PFMT did not persist until the 1-month follow-up. Besides, urinary incontinence improved significantly after 8 weeks of intervention, whereas the therapeutic effects did not persist in the 1-month follow-up of the concurrent sham M1 a-tDCS and PFMT group (sham group).
The majority of previous studies examining the effect of PFMT on the PFM function have reported significant improvements after a long period of exercise intervention in MS patients [5, 6, 29]. The results of these studies suggested that less than 8 weeks of training could not have any significant effects on the improvement of PFM function in patients with MS [5, 6, 29]. Moreover, Lúcio et al. investigated the effects of 12 weeks of PFMT (two sessions per week) on lower urinary tract disorders in 27 women with inflammation and reported fewer complaints of urinary incontinence symptoms, along with improvements in strength, endurance, resistance, and contraction rate of PFMs after training [6]. These findings were consistent with the results of the present study, as PFMT caused no significant improvements in a short period.
Additionally, Pérez et al. showed that a 12-week training program, focused on home-based PFMT, could significantly improve QOL, lower urinary tract symptoms, and the severity of urinary incontinence after the intervention in patients with MS, whereas no significant differences were observed in the 4th and 8th weeks of PFMT [5]. The results of the study by Pérez et al. confirmed the long-term effectiveness of PFMT, which is consistent with the present findings [5]. The results of a study by Ferreira et al. also showed the positive effects of 48-session PFMT along with perineal electrotherapy on the QOL, overactive bladder, perineal contraction, anxiety, and depression of patients with MS, which is consistent with the present results [29]. The findings of the present study also showed that PFMT for at least 8 weeks (a long-term intervention) could improve the PFM function, whereas there were no significant differences regarding the PFM function after 4-week PFMT.
There were some limitations to this pilot study. First, it was performed on a sample size of 30 patients; therefore, future studies with a larger sample size are suggested. Second, owing to time constraints, a 1-month follow-up period was considered in this study. It is recommended that future studies investigate the effects of concurrent M1 a-tDCS and PFMT in 6-month and 1-year follow-ups. Third, in this study, only female MS patients with an EDSS score <5.6 were evaluated; consequently, the findings cannot be generalized to severe MS patients. Future studies need to assess the effects of concurrent multi-session active M1 a-tDCS and PFMT on the PFM function and urinary incontinence of male and female patients with more severe MS.
Conclusion
According to the present results, concurrent active M1 a-tDCS and PFMT could significantly improve the PFM function by the 4th and 8th weeks of training and also in the 1-month follow-up after the intervention. The effects of concurrent sham M1 a-tDCS and PFMT were only significant in the 8th week of training, whereas there were no significant differences in the PFM function by the 4th week of training and at the 1-month follow-up. Moreover, the rate of urinary incontinence significantly reduced in both groups after the interventions. It is suggested that physiotherapists plan to employ concurrent active M1 a-tDCS and PFMT to enhance the effects of PFMT on the PFM function and prolong the efficacy of treatment for patients with MS.
References
Ayache SS, Lefaucheur J-P, Chalah MA. Long term effects of prefrontal tDCS on multiple sclerosis fatigue: a case study. Brain Stimul. 2017;10(5):1001–2.
Hosseinzadeh A, Baneshi MR, Sedighi B, Kermanchi J, Haghdoost AA. Incidence of multiple sclerosis in Iran: a nationwide, population-based study. Public Health. 2019;175:138–44.
Torad H, Shalaby N, Hussein HA, Sadek SZ, Abdelazim MS, Yehia A, et al. Bladder and urodynamic changes in multiple sclerosis. Egyptian J Neurol Psychiatr Neurosurg. 2020;56(1):47.
Ghezzi A, Carone R, Del Popolo G, Amato M, Bertolotto A, Comola M, et al. Recommendations for the management of urinary disorders in multiple sclerosis: a consensus of the Italian Multiple Sclerosis Study Group. Neurol Sci. 2011;32(6):1223–31.
Pérez DC, Chao CW, Jiménez LL, Fernández IM, De la Llave Rincon AI. Pelvic floor muscle training adapted for urinary incontinence in multiple sclerosis: a randomized clinical trial. Int Urogynecol J. 2020;31(2):267–75.
Lúcio AC, Campos RM, Perissinotto MC, Miyaoka R, Damasceno BP, D'ancona CAL. Pelvic floor muscle training in the treatment of lower urinary tract dysfunction in women with multiple sclerosis. Neurourol Urodyn. 2010;29(8):1410–3.
Mortezanejad M, Ehsani F, Masoudian N, Zoghi M, Jaberzadeh S. Comparing the effects of multi-session anodal trans-cranial direct current stimulation of primary motor and dorsolateral prefrontal cortices on fatigue and quality of life in patients with multiple sclerosis: a double-blind, randomized, sham-controlled trial. Clin Rehabil. 2020;34(8):1103–11.
Nardone R, Versace V, Sebastianelli L, Brigo F, Golaszewski S, Christova M, et al. Transcranial magnetic stimulation and bladder function: a systematic review. Clin Neurophysiol. 2019;130(11):2032–7.
Roy HA, Aziz TZ. Deep brain stimulation and multiple sclerosis: therapeutic applications. Mult Scler Relat Disord. 2014;3(4):431–9.
Benninger DH, Lomarev M, Lopez G, Pal N, Luckenbaugh DA, Hallett M. Transcranial direct current stimulation for the treatment of focal hand dystonia. Mov Disord. 2011;26(9):1698–702.
Ehsani F, Samai A. Effect of multi-session trans-cranial direct current stimulation of dorsolateral prefrontal area on depression and drowsiness in patients with multiple sclerosis. Koomesh. 2019;21(4):602–9.
Workman CD, Kamholz J, Rudroff T. Transcranial direct current stimulation (tDCS) to improve gait in multiple sclerosis: a timing window comparison. Front Hum Neurosci. 2019;13:420.
Chalah MA, Riachi N, Ahdab R, Mhalla A, Abdellaoui M, Créange A, et al. Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. J Neurol Sci. 2017;372:131–7.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302.
Klovning A, Avery K, Sandvik H, Hunskaar S. Comparison of two questionnaires for assessing the severity of urinary incontinence: the ICIQ-UI SF versus the incontinence severity index. Neurourol Urodyn. 2009;28(5):411–5.
Hajebrahimi S, Nourizadeh D, Hamedani R, Pezeshki MZ. Validity and reliability of the International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form and its correlation with urodynamic findings. Urol J. 2012;9(4):685–90.
Lee-Bognar E. Electrical stimulation is a useful adjunct in the management of urinary incontinence in people with multiple sclerosis. Aust J Physiother. 2009;55(1):62.
Ehsani F, Sahebi N, Shanbehzadeh S, Arab AM, ShahAli S. Stabilization exercise affects function of transverse abdominis and pelvic floor muscles in women with postpartum lumbo-pelvic pain: a double-blinded randomized clinical trial study. Int Urogynecol J. 2020;31(1):197–204.
Matsumoto M, Tsutaoka T, Yabunaka K, Handa M, Yoshida M, Nakagami G, et al. Development and evaluation of automated ultrasonographic detection of bladder diameter for estimation of bladder urine volume. PLoS One. 2019;14(9):e0219916.
Carrière B. Fitness for the pelvic floor. Stuttgart: Thieme. 2002. p 14–37.
George MS, Aston-Jones G. Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology. 2010;35(1):301–16.
Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–9.
Lim R, Liong ML, Lim KK, Leong WS, Yuen KH. The minimum clinically important difference of the international consultation on incontinence questionnaires (ICIQ-UI SF and ICIQ-LUTSqol). Urology. 2019;133:91–5.
Lattari E, Oliveira BRR, Monteiro Júnior RS, Marques Neto SR, Oliveira AJ, Maranhão Neto GA, et al. Acute effects of single dose transcranial direct current stimulation on muscle strength: a systematic review and meta-analysis. PLoS One. 2018;13(12):e0209513.
Allman C, Amadi U, Winkler AM, Wilkins L, Filippini N, Kischka U, et al. Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci Transl Med. 2016;8(330):330re1.
Hamoudi M, Schambra HM, Fritsch B, Schoechlin-Marx A, Weiller C, Cohen LG, et al. Transcranial direct current stimulation enhances motor skill learning but not generalization in chronic stroke. Neurorehabil Neural Repair. 2018;32(4–5):295–308.
Ehsani F, Ahmadi M, Masoudian N, Jaberzadeh S. Priming of postural training with cerebellar anodal transcranial direct current stimulation for its effects on postural balance and fear of falling in patients with multiple sclerosis: a randomized, double-blind, sham-controlled study. J Clin Neurosci. 2022;99:294–301.
Dutra LRDV, Silva-Filho E, Oliveira MC, Paiva Tavares BN, Pegado R, Micussi MTBAC. Transcranial direct current electrical stimulation for the treatment of interstitial cystitis: a study protocol. Eur J Obstet Gynecol Reprod Biol. 2021;262:198–202.
Ferreira APS, Pegorare ABGDS, Salgado PR, Casafus FS, Christofoletti G. Impact of a pelvic floor training program among women with multiple sclerosis: a controlled clinical trial. Am J Phys Med Rehabil. 2016;95(1):1–8.
Acknowledgements
We would like to thank the Neuromuscular Rehabilitation Research Center and Clinical Research and Development Unit of Kowsar Educational and Research and Therapeutic Center of Semnan University of Medical Sciences for providing facilities for this work.
Funding
This work was supported by the Neuromuscular Rehabilitation Research Center, Semnan University of Medical Sciences [Grant number: 1896].
Author information
Authors and Affiliations
Contributions
Mona Ramezani: protocol/project development, data collection, manuscript writing; Fatemeh Ehsani: protocol/project development, management, data analysis, manuscript writing; Cyrus Taghizadeh Delkhosh: data collection, manuscript writing; Nooshin Masoudian: data collection, management, data analysis, manuscript writing; Shapour Jaberzadeh: management, data analysis, manuscript editing.
Corresponding author
Ethics declarations
Conflicts of interest
Mona Ramezani, Fatemeh Ehsani, Cyrus Taghizadeh Delkhosh, Nooshin Masoudian, and Shapour Jaberzadeh declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
CONSORT checklist
(DOCX 570 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramezani, M., Ehsani, F., Delkhosh, C.T. et al. Concurrent multi-session anodal trans-cranial direct current stimulation enhances pelvic floor muscle training effectiveness for female patients with multiple sclerosis suffering from urinary incontinence and pelvic floor dysfunction: a randomized clinical trial study. Int Urogynecol J 34, 1771–1779 (2023). https://doi.org/10.1007/s00192-022-05429-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-022-05429-6